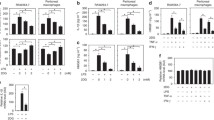
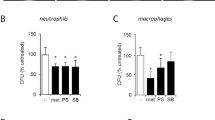
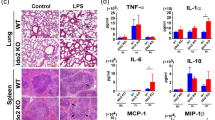
Abstract
Endotoxin shock remains one of the major causes of mortality worldwide. Pyruvate dehydrogenase kinase (PDK) 2 is an important regulatory enzyme involved in glucose metabolism. The purpose of this study was to determine the regulatory effect of PDK2 on LPS-induced endotoxin shock and explore the mechanisms in vivo and in vitro. Here, we showed that PDK2 contributed to Toll-like receptor (TLR)-mediated inflammation. Lipopolysaccharide (LPS) activation of TLR4 pathways resulted in PDK2 upregulation in macrophages and dendritic cells (DCs). PDK2 overexpression enhanced TLR4 signaling pathway activation, whereas downregulating PDK2 expression inhibited TLR4 signaling pathway activation. Pharmacological inhibition of PDK2 significantly decreased the mortality rate and alleviated pathological injury in the lungs and livers of LPS-challenged mice, while significantly suppressing proinflammatory cytokine production. Thus, we confirmed that PDK2 is involved in LPS-induced endotoxin shock by modulating TLR4-mitogen-activated protein kinase signaling and inducing the production of proinflammatory cytokines in macrophages and DCs. Our findings highlight the importance of PDK2 as a novel target to treat septic shock.







Similar content being viewed by others
AVAILABILITY OF DATA AND MATERIALS
Data that support the results of the present study are available from the corresponding author upon reasonable request.
References
Singer, M., C.S. Deutschman, C.W. Seymour, M. Shankar-Hari, D. Annane, M. Bauer, R. Bellomo, G.R. Bernard, J.D. Chiche, C.M. Coopersmith, R.S. Hotchkiss, M.M. Levy, J.C. Marshall, G.S. Martin, S.M. Opal, G.D. Rubenfeld, T. van der Poll, J.L. Vincent, and D.C. Angus. 2016. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315: 801–810. https://doi.org/10.1001/jama.2016.0287.
Font, M.D., B. Thyagarajan, and A.K. Khanna. 2020. Sepsis and septic shock - basics of diagnosis, pathophysiology and clinical decision making. Medical Clinics of North America 104: 573–585. https://doi.org/10.1016/j.mcna.2020.02.011.
Liu, Z., Y. Fan, Y. Wang, C. Han, Y. Pan, H. Huang, Y. Ye, L. Luo, and Z. Yin. 2008. Dipyrithione inhibits lipopolysaccharide-induced iNOS and COX-2 up-regulation in macrophages and protects against endotoxic shock in mice. FEBS letters 582: 1643–1650. https://doi.org/10.1016/j.febslet.2008.04.016.
de Padua Lucio K., A.C.S. Rabelo, C.M. Araujo, G.C. Brandao, G.H.B. de Souza, R.G. da Silva, D.M.S. de Souza, A. Talvani, F.S. Bezerra, A.J. Cruz Calsavara, and D.C. Costa. 2018. Anti-inflammatory and antioxidant properties of black mulberry (Morus nigra L.) in a model of LPS-induced sepsis. Oxidative medicine and cellular longevity 2018:5048031. https://doi.org/10.1155/2018/5048031.
Napier, B.A., M. Andres-Terre, L.M. Massis, A.J. Hryckowian, S.K. Higginbottom, K. Cumnock, K.M. Casey, B. Haileselassie, K.A. Lugo, D.S. Schneider, J.L. Sonnenburg, and D.M. Monack. 2019. Western diet regulates immune status and the response to LPS-driven sepsis independent of diet-associated microbiome. Proceedings of the National Academy of Sciences of the United States of America 116: 3688–3694. https://doi.org/10.1073/pnas.1814273116.
Delano, M.J., and P.A. Ward. 2016. Sepsis-induced immune dysfunction: Can immune therapies reduce mortality? The Journal of clinical investigation 126: 23–31. https://doi.org/10.1172/JCI82224.
Kolaczkowska, E., M. Lelito, E. Kozakiewicz, N. van Rooijen, B. Plytycz, and B. Arnold. 2007. Resident peritoneal leukocytes are important sources of MMP-9 during zymosan peritonitis: Superior contribution of macrophages over mast cells. Immunology letters 113: 99–106. https://doi.org/10.1016/j.imlet.2007.07.017.
Li, X., X. Yao, Y. Zhu, H. Zhang, H. Wang, Q. Ma, F. Yan, Y. Yang, J. Zhang, H. Shi, Z. Ning, J. Dai, Z. Li, C. Li, F. Su, Y. Xue, X. Meng, G. Dong, and H. Xiong. 2019. The caspase inhibitor Z-VAD-FMK alleviates endotoxic shock via inducing macrophages necroptosis and promoting MDSCs-mediated inhibition of macrophages activation. Frontiers in immunology 10: 1824. https://doi.org/10.3389/fimmu.2019.01824.
Shin, J.S., K.G. Lee, H.H. Lee, H.J. Lee, H.J. An, J.H. Nam, D.S. Jang, and K.T. Lee. 2016. alpha-Solanine Isolated From Solanum Tuberosum L. cv Jayoung Abrogates LPS-induced inflammatory responses via NF-kappaB inactivation in RAW 264.7 macrophages and endotoxin-induced shock model in mice. Journal of cellular biochemistry 117: 2327–2339. https://doi.org/10.1002/jcb.25530.
Medzhitov, R., C.A. Janeway, and Jr. 1997. Innate immunity: The virtues of a nonclonal system of recognition. Cell 91: 295–298. https://doi.org/10.1016/s0092-8674(00)80412-2.
Pearce, E.L., M.C. Poffenberger, C.H. Chang, and R.G. Jones. 2013. Fueling immunity: Insights into metabolism and lymphocyte function. Science 342: 1242454. https://doi.org/10.1126/science.1242454.
Pearce, E.L., and E.J. Pearce. 2013. Metabolic pathways in immune cell activation and quiescence. Immunity 38: 633–643. https://doi.org/10.1016/j.immuni.2013.04.005.
McGettrick, A.F., and L.A. O’Neill. 2013. How metabolism generates signals during innate immunity and inflammation. Journal of Biological Chemistry 288: 22893–22898. https://doi.org/10.1074/jbc.R113.486464.
Roche, T.E., and Y. Hiromasa. 2007. Pyruvate dehydrogenase kinase regulatory mechanisms and inhibition in treating diabetes, heart ischemia, and cancer. Cellular and Molecular Life Sciences 64: 830–849. https://doi.org/10.1007/s00018-007-6380-z.
Rahman, M.H., A. Bhusal, J.H. Kim, M.K. Jha, G.J. Song, Y. Go, I.S. Jang, I.K. Lee, and K. Suk. 2020. Astrocytic pyruvate dehydrogenase kinase-2 is involved in hypothalamic inflammation in mouse models of diabetes. Nature Communications 11: 5906. https://doi.org/10.1038/s41467-020-19576-1.
Go, Y., J.Y. Jeong, N.H. Jeoung, J.H. Jeon, B.Y. Park, H.J. Kang, C.M. Ha, Y.K. Choi, S.J. Lee, H.J. Ham, B.G. Kim, K.G. Park, S.Y. Park, C.H. Lee, C.S. Choi, T.S. Park, W.N. Lee, R.A. Harris, and I.K. Lee. 2016. Inhibition of pyruvate dehydrogenase kinase 2 protects against hepatic steatosis through modulation of tricarboxylic acid cycle anaplerosis and ketogenesis. Diabetes 65: 2876–2887. https://doi.org/10.2337/db16-0223.
Sun H., A. Zhu, X. Zhou, F. Wang. 2017. Suppression of pyruvate dehydrogenase kinase-2 re-sensitizes paclitaxel-resistant human lung cancer cells to paclitaxel. Oncotarget 8:52642–52650. https://doi.org/10.18632/oncotarget.16991.
He, Z., Z. Li, X. Zhang, K. Yin, W. Wang, Z. Xu, B. Li, L. Zhang, J. Xu, G. Sun, L. Wang, Q. Li, X. Huang, L. Zhang, D. Zhang, H. Xu, and Z. Xu. 2018. MiR-422a regulates cellular metabolism and malignancy by targeting pyruvate dehydrogenase kinase 2 in gastric cancer. Cell Death & Disease 9: 505. https://doi.org/10.1038/s41419-018-0564-3.
Yagi, Y., M. Kuwahara, J. Suzuki, Y. Imai, and M. Yamashita. 2020. Glycolysis and subsequent mevalonate biosynthesis play an important role in Th2 cell differentiation. Biochemical and Biophysical Research Communications 530: 355–361. https://doi.org/10.1016/j.bbrc.2020.08.009.
Min, B.K., S. Park, H.J. Kang, D.W. Kim, H.J. Ham, C.M. Ha, B.J. Choi, J.Y. Lee, C.J. Oh, E.K. Yoo, H.E. Kim, B.G. Kim, J.H. Jeon, D.Y. Hyeon, D. Hwang, Y.H. Kim, C.H. Lee, T. Lee, J.W. Kim, Y.K. Choi, K.G. Park, A. Chawla, J. Lee, R.A. Harris, and I.K. Lee. 2019. Pyruvate dehydrogenase kinase is a metabolic checkpoint for polarization of macrophages to the M1 phenotype. Frontiers in immunology 10: 944. https://doi.org/10.3389/fimmu.2019.00944.
Na, Y.R., D. Jung, J. Song, J.W. Park, J.J. Hong, and S.H. Seok. 2020. Pyruvate dehydrogenase kinase is a negative regulator of interleukin-10 production in macrophages. Journal of Molecular Cell Biology 12: 543–555. https://doi.org/10.1093/jmcb/mjz113.
Meyers, A.K., Z. Wang, W. Han, Q. Zhao, M. Zabalawi, J. Liu, R.K. Manne, H.K. Lin, C.M. Furdui, J.W. Locasale, and C.M. McCall. 2021. Pyruvate dehydrogenase kinase supports macrophage NLRP3 inflammasome activation during acute inflammation.
Tripathi, S., D. Bruch, and D.S. Kittur. 2008. Ginger extract inhibits LPS induced macrophage activation and function. BMC Complementary and Alternative Medicine 8: 1. https://doi.org/10.1186/1472-6882-8-1.
Morrell, J.A., J. Orme, R.J. Butlin, T.E. Roche, R.M. Mayers, and E. Kilgour. 2003. AZD7545 is a selective inhibitor of pyruvate dehydrogenase kinase 2. Biochemical Society transactions 31: 1168–1170. https://doi.org/10.1042/bst0311168.
Mayers, R.M., R.J. Butlin, E. Kilgour, B. Leighton, D. Martin, J. Myatt, J.P. Orme, and B.R. Holloway. 2003. AZD7545, a novel inhibitor of pyruvate dehydrogenase kinase 2 (PDHK2), activates pyruvate dehydrogenase in vivo and improves blood glucose control in obese (fa/fa) Zucker rats. Biochemical Society transactions 31: 1165–1167. https://doi.org/10.1042/bst0311165.
Cesi, G., G. Walbrecq, A. Zimmer, S. Kreis, and C. Haan. 2017. ROS production induced by BRAF inhibitor treatment rewires metabolic processes affecting cell growth of melanoma cells. Molecular cancer 16: 102. https://doi.org/10.1186/s12943-017-0667-y.
van der Poll, T., F.L. van de Veerdonk, B.P. Scicluna, and M.G. Netea. 2017. The immunopathology of sepsis and potential therapeutic targets. Nature Reviews Immunology 17: 407–420. https://doi.org/10.1038/nri.2017.36.
Prescott, H.C., and D.C. Angus. 2018. Enhancing recovery from sepsis: A review. JAMA 319: 62–75. https://doi.org/10.1001/jama.2017.17687.
Kaukonen, K.M., M. Bailey, S. Suzuki, D. Pilcher, and R. Bellomo. 2014. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 311: 1308–1316. https://doi.org/10.1001/jama.2014.2637.
Rezinciuc, S., L. Bezavada, A. Bahadoran, S. Duan, R. Wang, D. Lopez-Ferrer, D. Finkelstein, M.A. McGargill, D.R. Green, L. Pasa-Tolic, and H.S. Smallwood. 2020. Dynamic metabolic reprogramming in dendritic cells: An early response to influenza infection that is essential for effector function. PLoS Pathogens 16: e1008957. https://doi.org/10.1371/journal.ppat.1008957.
Soto-Heredero, G., Gomez de Las, M.M. Heras, E. Gabande-Rodriguez, J. Oller, and M. Mittelbrunn. 2020. Glycolysis - a key player in the inflammatory response. The FEBS journal 287: 3350–3369. https://doi.org/10.1111/febs.15327.
Bajotto, G., T. Murakami, M. Nagasaki, B. Qin, Y. Matsuo, K. Maeda, M. Ohashi, Y. Oshida, Y. Sato, and Y. Shimomura. 2006. Increased expression of hepatic pyruvate dehydrogenase kinases 2 and 4 in young and middle-aged Otsuka Long-Evans Tokushima Fatty rats: Induction by elevated levels of free fatty acids. Metabolism 55: 317–323. https://doi.org/10.1016/j.metabol.2005.09.014.
Arora, H., S.M. Wilcox, L.A. Johnson, L. Munro, B.A. Eyford, C.G. Pfeifer, I. Welch, and W.A. Jefferies. 2019. The ATP-binding cassette gene ABCF1 functions as an E2 ubiquitin-conjugating enzyme controlling macrophage polarization to dampen lethal septic shock. Immunity 50 (418–431): e416. https://doi.org/10.1016/j.immuni.2019.01.014.
Fan, M., X. Li, X. Gao, L. Dong, G. Xin, L. Chen, J. Qiu, and Y. Xu. 2019. LPS Induces preeclampsia-like phenotype in rats and HTR8/SVneo cells dysfunction through TLR4/p38 MAPK pathway. Frontiers in Physiology 10: 1030. https://doi.org/10.3389/fphys.2019.01030.
de Oliveira, R.G., Castilho G. R. de Campos, A.L. da Cunha, F. Miyajima, and Martins D. T. de Oliveira. 2017. Dilodendron bipinnatum Radlk. inhibits pro-inflammatory mediators through the induction of MKP-1 and the down-regulation of MAPKp38/JNK/NF-kappaB pathways and COX-2 in LPS-activated RAW 264.7 cells. Journal of Ethnopharmacology 202: 127–137. https://doi.org/10.1016/j.jep.2017.02.026.
ACKNOWLEDGEMENTS
We thank the laboratory students at Jining Medical University (Lina Jing, Dalei Cheng, Hongyan Cheng, and Chenyu Li) for their helpful contribution under the guidance of Huabao Xiong. We also thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81874169); the Shandong Provincial Natural Science Foundation, China (No. ZR2020MH163 and ZR2020KH033); the Project of the Medical and Health Technology Development Program in Shandong Province, China (No. 2019WS356); the Research Fund for Lin He’s Academician Workstation of New Medicine and Clinical Translation in Jining Medical University (No. JYHL2021MS06); and the NSFC Cultivation Project of Jining Medical University, China (No. JYP2019KJ23).
Author information
Authors and Affiliations
Contributions
Huabao Xiong, Chunxia Li, Jun Dai, and Guanjun Dong contributed to study concept and design. Huabao Xiong directed the study, contributed to data interpretation, and drafted and critically revised the manuscript. Huabao Xiong, Chunxia Li, Jun Dai, and Junfeng Zhang obtained funding. Chunxia Li, Chuanbin Liu, Xin Zhang, and Junfeng Zhang performed all experiments in vitro and carried out primary analyses. All other experiments and mouse manipulation were performed by Chunxia Li, Zhaochen Ning, Qun Ma, and Zhihua Li. Fenglian Yan and Hui Zhang carried out histopathological interpretation. Changying Wang and Mingsheng Zhao carried out FACS-adjusted and compositional analysis. Chunxia Li, Chuanbin Liu, Guanjun Dong, and Hui Shi contributed to manuscript preparation and data analyses. Jun Dai and Chunxia Li contributed equally to this work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the Jining University Institution Animal Care and Use Committee (No. jnmc-2019-zr-0012).
Consent to Participate
Not applicable.
Consent for Publication
All authors have approved the manuscript and agreed with the submission.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Dai, J., Liu, C. et al. Pyruvate Dehydrogenase Kinase 2 Accelerates Endotoxin Shock by Promoting Mitogen-Activated Protein Kinase Activation. Inflammation 46, 418–431 (2023). https://doi.org/10.1007/s10753-022-01744-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01744-8