Abstract
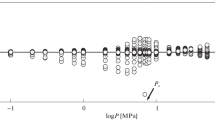
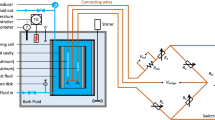
The thermal conductivities of carbon dioxide and three mixtures of carbon dioxide and methane at six nominal temperatures between 300 and 425 K have been measured as a function of pressure up to 12 MPa. The measurements were made with a transient hot-wire apparatus. The relative uncertainty of the reported thermal conductivities at a 95% confidence level is estimated to be ±1.2%. Results of the low-density analysis of the obtained data were used to test expressions for predicting the thermal conductivity of nonpolar mixtures in a dilute-gas limit developed by Schreiber, Vesovic, and Wakeham. The scheme was found to underestimate the experimental thermal conductivity with deviations not exceeding 5%. The dependence of the thermal conductivity on density was used to test the predictive scheme for the thermal conductivity of gas mixtures under pressure suggested by Mason et al. and improved by Vesovic and Wakeham. Comparisons reveal a pronounced critical enhancement on isotherms at 300 and 325 K for mixtures with methane mole fractions of 0.25 and 0.50. For other states, comparisons of the experimental and predicted excess thermal conductivity contributions showed a smaller increase of the experimental data with deviations approaching 3% within the examined range of densities.
Similar content being viewed by others
References
M. Schreiber V. Vesovic W.A. Wakeham (1997) Int. J. Thermophys. 18 925
M.J. Ross V. Vesovic W.A. Wakeham (1992) Physica A 183 519
E.A. Mason H.E. Khalifa J. Kestin R. DiPippo J.R. Dorfman (1978) Physica A 91 377
J. Kestin W.A. Wakeham (1980) Ber. Bunsenges Phys. Chem. 84 762
V. Vesovic W.A. Wakeham (1991) High Temp. High Press. 23 179
M.K. Tham K.E. Gubbins (1971) J. Chem. Phys. 55 268 Occurrence Handle10.1063/1.1675518
J.O. Hirschfelder (1957) J. Chem. Phys. 26 282 Occurrence Handle10.1063/1.1743285
J. Luettmer-Strathmann J.V. Sengers (1994) Int. J. Thermophys. 15 1421 Occurrence Handle10.1007/BF01458832
J. Luettmer-Strathmann J.V. Sengers (1996) J. Chem. Phys. 104 3026 Occurrence Handle10.1063/1.471070
J. Pátek J. Klomfar L. Čapla P. Buryan (2003) Int. J. Thermophys. 24 923 Occurrence Handle10.1023/A:1025024127880
B.M. Rosenbaum G. Thodos (1969) J. Chem Phys. 51 1361 Occurrence Handle10.1063/1.1672182
J. Kestin S.T. Ro Y. Nagasaka (1982) Ber. Bunsenges Phys. Chem. 86 945
J. Pátek J. Klomfar (2002) Fluid Phase Equilib. 198 147 Occurrence Handle10.1016/S0378-3812(01)00763-4
R. Span W. Wagner (1996) J. Phys. Chem. Ref. Data 15 1509
Compressibility Factors of Natural Gas and Other Related Hydrocarbon Gases, A.G.A. Report No. 8, Second Ed (Arlington, Virginia, 1992).
ISO 12 213, Natural Gas–Calculation of Compression Factor: Calculation using Molar–Composition Analysis. First Ed., (Geneva, 1997).
M. Bureš R. Holub J. Leitner P. Vonka (1992) The Thermochemical Quantities of Organic Compounds Institute of Chemical Technology Editing Center Prague
Taylor B.N., Kyatt Ch.E. Guidelines for Evaluating and Expressing the Uncertainty of NIST Measurement Results, NIST Tech. Note 1297 (1994).
V. Vesovic W.A. Wakeham G.A. Olchowy J.V Sengers J.T.R Watson J. Millat (1990) J. Phys. Chem. Ref. Data 19 763
M.J. Assael J. Millat V. Vesovic W.A Wakeham (1990) J. Phys. Chem. Ref. Data 19 1137
V. Vesovic (2000) High Temp. High Press. 32 163
V. Vesovic (2001) Int. J. Thermophys. 22 801 Occurrence Handle10.1023/A:1010727016194
D.G. Friend J.F. Ely H. Ingham (1989) J. Phys Chem. Ref. Data 18 583
G.C. Maitland E.B. Smith M. Rigby W.A Wakeham (1981) Intermolecular Forces Claredon Press Oxford
J. Millat M. Mustafa M. Ross W.A. Wakeham M. Zalaf (1987) Physica A 145 461
A.I. Johns S. Rashid J.T.R Watson A.A Clifford (1986) J. Chem. Soc. Faraday Trans. 1 82 2235 Occurrence Handle10.1039/f19868202235
A. Scott A.I. Johns J.T.R Watson A.A Clifford (1983) J. Chem. Soc. Faraday Trans. 1 79 733 Occurrence Handle10.1039/f19837900733
A.A. Clifford J. Kestin W.A. Wakeham (1979) Physica A 97 287
J.M. Lenoir E.W. Comings (1951) Chem. Eng Prog. 47 223
H.L. Johnston E.R. Grilly (1946) J. Chem Phys. 14 233 Occurrence Handle10.1063/1.1724125
B.G. Dikins (1934) Proc. Roy. Soc. Lond. A 143 517
J. Kestin Y. Nagasaka W.A. Wakeham (1982) Ber. Bunsenges. Phys. Chem. 86 632
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pátek, J., Klomfar, J., Čapla, L. et al. Thermal Conductivity of Carbon Dioxide–Methane Mixtures at Temperatures Between 300 and 425 K and at Pressures up to 12 MPa. Int J Thermophys 26, 577–592 (2005). https://doi.org/10.1007/s10765-005-5566-6
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10765-005-5566-6