Abstract
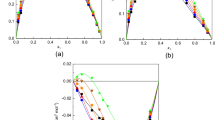
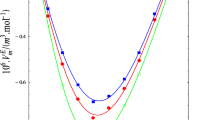
Speeds of sound and densities of 2-propanol +1-propanol, 2-propanol + 1-butanol, 2-propanol + 1-octanol, and 2-propanol + 1-hexanol have been measured over the entire composition range at 298.15 K. Speeds of sound of the binary mixtures have also been estimated from free length theory (FLT), collision factor theory (CFT), and Nomoto’s relation (NR) and have been compared with experimental speeds of sound. The isentropic compressibilities, molar isentropic compressibilities, excess molar isentropic compressibilities, and excess speeds of sound have been calculated from experimental densities and speeds of sound. Excess molar isentropic compressibilities and excess speeds of sound of the binary mixtures were fitted to the Redlich–Kister equation
Similar content being viewed by others
References
O. Redlich A.T. Kister (1948) Ind. Eng. Chem 40 345 Occurrence Handle10.1021/ie50458a036
B. Jacobson (1952) J. Chem. Phys 6 927 Occurrence Handle10.1063/1.1700615
B. Jacobson (1952) Acta. Chem. Scand 6 1485
W. Schaafs (1963) Molekularakustik Springer-Verlag Berlin, Göttingen, Heidelberg, Germany
O. Nomoto (1958) J. Phys. Soc 13 1528 Occurrence Handle10.1143/JPSJ.13.1528
G. Savaroglu E. Aral (2004) Fluid Phase Equilib. 215 253 Occurrence Handle10.1016/j.fluid.2003.09.001
J.A. Riddich W.B. Bunger T.K. Sokano (1986) Organic Solvents Physical Properties and Methods of Purification (Techniques of Chemistry) Vol. 2 EditionNumber4 Wiley/Interscience New York
T.M. Letcher N. Deenadayalu (2000) J. Chem. Eng Data 45 730 Occurrence Handle10.1021/je000043v
C.A. Cerdeirina C.A. Tovar J. Troncoso E. Carballo L. Romani (1999) Fluid Phase Equilib. 157 93 Occurrence Handle10.1016/S0378-3812(99)00018-7
A. Valen M.C. Lopez J.S. Urieta F.M. Royo C. Lafuente (2002) J. Mol. Liquids 95 157 Occurrence Handle10.1016/S0167-7322(01)00279-3
T.P. Iglesias J.L. Legido L. Romani M.I. Paz Andrade (1993) Phys. Chem. Liq 25 135
E. Mascato L. Mosteiro M.M. Pineiro J. Garcia T.P. Iglesias J.L. Legido (2001) J. Chem. Thermodyn 33 1081
A. Heintz B. Schmittecker D. Wagner R.N. Lichtenthaler (1986) J. Chem. Eng. Data 31 487 Occurrence Handle10.1021/je00046a030
A. Arce A. Arce SuffixJr. J. Artinez-Ageitos E. Rodil O. Rodriguez (2000) Fluid Phase Equilib. 170 113 Occurrence Handle10.1016/S0378-3812(00)00328-9
T.M. Aminabhavi M.I. Aralaguppi S.B. Harogoppad R.H. Balundgi (1993) J. Chem. Eng. Data 38 31 Occurrence Handle10.1021/je00009a008
C. Gonzalez M. Iglesias J. Lanz G. Marino B. Orge J.M. Resa (2001) J Food Eng. 50 29 Occurrence Handle10.1016/S0260-8774(00)00192-8
O. Kiyohara G.C. Benson (1981) J Solution Chem. 10 281 Occurrence Handle10.1007/BF00645017
B.E. Cominges Particlede M.M. Pineiro T.P. Iglesias J.L. Legido M.I. Paz Andrade (1998) J. Chem Thermodyn. 30 1147 Occurrence Handle10.1006/jcht.1998.0382
S.L. Oswal S.S.R. Putta (2001) Thermochim Acta 373 141 Occurrence Handle10.1016/S0040-6031(00)00778-4
A. Pineiro A. Amigo R. Bravo P. Brocos (2000) Fluid Phase Equilib. 173 211 Occurrence Handle10.1016/S0378-3812(00)00431-3
A. Pineiro P. Brocos A. Amigo M. Pintos (2002) J. Solution Chem 31 369 Occurrence Handle10.1023/A:1015807331250
CDATA, Database of Thermodynamic and Transport Properties for Chemistry and Engineering, Version 1.020 (Department of Physical Chemistry, Institute of Chemical Technology, Prague, Czech Republic, 1999).
E. Calvo P. Brocos A. Pineiro M. Pintos A. Amigo R. Bravo A.H. Roux-Desgranges (1999) J. Chem. Eng. Data 44 948 Occurrence Handle10.1021/je990078z
G. Douheeret A. Pal M.I. Davis (1990) J Chem Thermodyn 22 99 Occurrence Handle10.1016/0021-9614(90)90036-P
G. Douheeret A. Pal M.I. Davis J. Loya (1992) Thermochim. Acta 207 313 Occurrence Handle10.1016/0040-6031(92)80145-M
G. Douheeret M.I. Davis J.C.R. Reis M.J. Blandamer (2001) Chem. Phys. Chem 2 148
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Savaroglu, G., Aral, E. Speeds of Sound and Isentropic Compressibilities in Binary Mixtures of 2-Propanol with Several 1-Alkanols at 298.15 K. Int J Thermophys 26, 1525–1535 (2005). https://doi.org/10.1007/s10765-005-8101-x
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10765-005-8101-x