Abstract
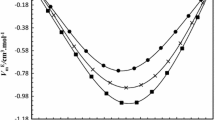
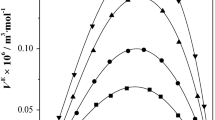
This paper presents experimental data for densities, ρ, ultrasonic velocities, u, and refractive indices, n, of pure dimethylsulphoxide (DMSO), 1-butanol, 1-hexanol, 1-heptanol, and their binary mixtures, with DMSO as a common component, over the whole composition range at 303.15 K. The molar refraction, Rm, molecular association, MA, excess molar volume, VE, and deviation in isentropic compressibility, ΔKs, were calculated from the experimental data. The apparent molar volume, Vϕ,2, and apparent molar isentropic compressibility, Kϕ,2, of alkanols in DMSO were also calculated. The values of Vϕ,2 and Kϕ,2 were used to estimate the partial molar volume, \(\bar{V}^{0}_{\phi,2}\), and partial molar isentropic compressibility, \(\bar{K}^{0}_{\phi,2}\), of alkanols in DMSO at infinite dilution. The changes in these parameters with composition and the size of the alkyl chain length in the alkanol molecule are discussed with reference to the nature of interactions between component molecules. Excess molar volumes have also been estimated from measurements of refractive indices
Similar content being viewed by others
References
A. Ali Abida S. Hyder (2004) Phys Chem Liq. 42 411 Occurrence Handle10.1080/00319100410001697864
A. Ali Abida A.K. Nain S. Hyder (2003) J. Solution Chem 32 865 Occurrence Handle10.1023/B:JOSL.0000013430.02702.fa
A. Ali Abida A.K. Nain S. Hyder (2002) Collect Czech. Chem. Commun 67 1125 Occurrence Handle10.1135/cccc20021125
C.D. Edas (2000) J. Phys. Chem. B 104 6653 Occurrence Handle10.1021/jp993472c
A.J. Parker (1969) Chem. Rev 16 163
InstitutionalAuthorNameCrown Zellerbach Corp. (1968) Dimethylsulphoxide as a Reaction Solvent Camas Washington
S.W. Jacob E.E. Rosenbaum D.C. Wood (1971) Dimethylsulphoxide Marcel Dekker New York
J.T. Lal F.W. Lau D. Robb P. Westh G. Nielsein C. Trandum A. Hvidt Y. Koga (1995) J. Solution Chem. 24 89 Occurrence Handle10.1007/BF00973051
R.C. Scaduto (1995) Free Radical Biol. Med 18 271 Occurrence Handle10.1016/0891-5849(94)E0139-A
S.T. Osinska (1993) Chem. Soc. Rev 22 205 Occurrence Handle10.1039/cs9932200205
A.F. Fucaloro (2002) J. Chem. Edu 79 865
A.I. Vogel (1989) Text Book of Practical Organic Chemistry EditionNumber5 Longmans Green London
R.H. Stokes (1965) Viscosity of Electrolytes and Related Properties Pergamon Press Oxford
J. Ortega (1982) J. Chem Eng Data 27 312 Occurrence Handle10.1021/je00029a024
K. Tiwari C. Patra S. Padhy V. Chakravortty (1996) Phys Chem Liq. 32 149
A. Ali K. Tiwari A.K. Nain V. Chakravortty (2000) Phys. Chem. Liq 38 459
S.L. Oswal K.D. Prajapati N.Y. Ghael S.P. Ijardar (2004) Fluid Phase Equilib. 218 131 Occurrence Handle10.1016/j.fluid.2003.11.012
G. Ritzoulis (1989) Can. J. Chem. 67 1105
A. Ali S. Hyder A.K. Nain (1999) J. Mol. Liq. 79 89 Occurrence Handle10.1016/S0167-7322(98)00105-6
Van Dael W., Vangeel E. Proc. Ist Int. Conf. Calorimetry Thermodyn., Warsaw (1969).
W.S. Brey (1978) Physical Chemistry and Biological Applications Academic Press New York
Sjoblom J., Dyhr H., Hansen O. Finn. Chem. Lett. 110 (1981).
S.K. Mehta R.K. Chauhan R.K. Dewan (1992) J. Chem. Soc Faraday Trans. 92 1167 Occurrence Handle10.1039/ft9969201167
O. Redlich A.T. Kister (1948) Ind. Eng. Chem. 40 345 Occurrence Handle10.1021/ie50458a036
Fort R.J., Moore W.R. Trans Faraday Soc. 61:2102 (1965); 62:1112 (1966).
Ali A., Hyder S., Nain A.K. Acoustics Lett. 21:77 (1997); 23:183 (2000).
A. Pal S. Sharma H. Kumar (2003) J. Mol. Liq. 108 231 Occurrence Handle10.1016/S0167-7322(03)00184-3
B. Hawrylak K. Gracie R. Palepu (1998) J. Solution Chem 27 17 Occurrence Handle10.1023/A:1022636511542
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, A., Hyder, S. & Tariq, M. Measurements of the Properties of Binary Mixtures of Dimethylsulphoxide (DMSO) with 1-Alkanols (C4, C6, C7) at 303.15 K. Int J Thermophys 26, 1537–1548 (2005). https://doi.org/10.1007/s10765-005-8102-9
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10765-005-8102-9