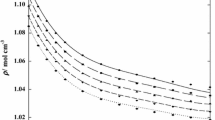
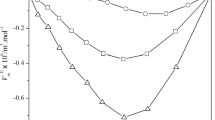
Densities and absolute viscosities for the binary mixtures containing tetrahydropyran and isomeric chlorobutanes have been determined at 298.15 and 313.15 K at atmospheric pressure. From the densities, excess molar volumes were calculated. On the other hand, excess volumes and absolute viscosities have been correlated using the Peng–Robinson–Stryjek–Vera (PRSV) equation of state and the Lee model, respectively.
Similar content being viewed by others
References
Reyes A, Haro M, Gascón I, Artigas H, Lafuente C (2003). J. Chem. Eng. Data 48:887
Giner B, Aldea M.E, Martín S., Gascón I, Lafuente C, (2003). J. Chem. Eng. Data 48:1296
Giner B, Reyes A, Haro M, Martín S., Royo F.M, (2004). Phys. Chem. Liquids 42:173
Giner B, Villares A, Gascón I., Cea P, Lafuente C, (2004). Int. J. Thermophys. 25:1735
Aldea M.E, Giner B, Gascón I., Artigas H, Villares A, Lafuente C, (2005). Thermochim. Acta 429:233
Giner B, Gascón I., Artigas H, Villares A, Lafuente C, (2006). J. Thermal Anal. Calorim. 83:735
Giner B, Artigas H, Haro M, Lafuente C, and M. C. López, J. Mol. Liquids (in press).
Kijeveanin M.L, Djordjević B.D., Žerbanović S.P., Grgurić I.R., Tasić A.Ž., (2004). Phys. Chem. Liquids 42:147
García B., Aparicio S, Navarro A.M, Alcalde R, Leal J.M, (2004). J. Phys. Chem. B 108:15841
Peng D.Y., Robinson D.B, (1976). Ind. Eng. Chem. Fundam. 15:59
Stryjek R., Vera J.H, (1986). Can. J. Chem. Eng. 64:32
Lee L., Lee Y, (2001). Fluid Phase Equilib. 181:47
Inglese A, J. Grolier P.E, Wilhelm E, (1984). Fluid Phase Equilib. 15:287
Riddick J.A, Bunger W.B, Sakano T.K, (1986). Organic Solvents. Physical Properties and Methods of Purification (Techniques of Chemistry, Vol. II) 3rd edn. Wiley-Interscience, New York
Carvajal C, Tölle K.S., Smid J, Szwarc M, (1965). J. Am. Chem. Soc. 87:5548
Timmermans J, (1965). Physico-Chemical Constants of Pure Organic Compounds, Vol II. Elsevier, Amsterdam
Giner B, López M.C., Cea P, Lafuente C, Royo F.M, (2005). Fluid Phase Equilib. 232:50
Poling B.E, Prausnitz J.M, O’Connell J.P., (2001). The Properties of Gases and Liquids, 5th edn. McGraw-Hill, New York
Constantinou L., Gani R, (1994). AIChE J. 40:1697
Arnaud J.F, Ungerer P, Behar E, Moracchini B, Sánchez J., (1996). Fluid Phase Equilib. 124:177
Valderrama J.O, (2003). Ind. Eng. Chem. Res. 42:1603
Marquardt D.W, (1963). J. Soc. Ind. Appl. Math. 11:431
Glasstone S.K, Laidler J, Eyring H, (1941). The Theory of Rate Processes. McGraw-Hill, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giner, B., Gascón, I., Artigas, H. et al. Thermophysical Properties of Mixtures of Tetrahydropyran with Chlorobutanes. Int J Thermophys 27, 1406–1418 (2006). https://doi.org/10.1007/s10765-006-0100-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-006-0100-z