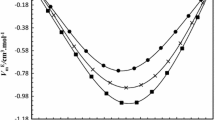
The densities of binary mixtures of aniline with 1-propanol, 2-propanol, 2-methyl-1-propanol, and 2-methyl-2-propanol were measured over the entire composition range, along with the pure components, at temperatures of 293.15, 298.15, 303.15, 308.15, 313.15, and 318.15 K and atmospheric pressure. Using the experimental data, the excess molar volumes, \(V_{\rm m}^{\rm E}\) , and the temperature coefficients of the excess molar volume, \(\partial V_{\rm m}^{\rm E}/\partial T\) for the binary mixtures were calculated. The variations of these parameters with composition and temperature of the mixtures have been discussed in terms of molecular interactions in these mixtures. The \(V_{\rm m}^{\rm E}\) values were found negative for all the mixtures at each temperature studied, indicating the presence of specific interactions between aniline and alkanol molecules. The extent of negative deviations in \(V_{\rm m}^{\rm E}\) values follows the order: 1-propanol < 2-propanol < 2-methyl-1-propanol < 2-methyl-2-propanol. It is observed that the \(V_{\rm m}^{\rm E}\) values depend upon the positions of hydroxyl and methyl groups in these alkanol molecules.
Similar content being viewed by others
References
Kinart C.M., Kinart W.J., Checinska-Majak D., Cwiklinska A. (2004). J. Mol. Liq. 109:19
Oswal S.L., Desai H.S. (1998). Fluid Phase Equilib. 149:359
Garcia B., Alcalde R., Leal J.M., Matos J.S. (1996). J. Chem. Soc., Faraday Trans. 92:3347
Nain A.K. (2006). J. Chem. Thermodyn. 38:1362
Nain A.K. (2006). J. Solution Chem. 35:1417
Nain A.K. (2006). Bull. Chem. Soc. Jpn. 79:1688
Ali A., Nain A.K. (2002). Bull. Chem. Soc. Jpn. 75:681
Nain A.K., Ali A., Alam M. (1998). J. Chem. Thermodyn. 30:1275
Ali A., Chand D., Nain A.K., Ahmad R. (2006). Int. J. Thermophys. 27:1482
Ali A., Nain A.K., Chand D., Ahmad R. (2006). Bull. Chem. Soc. Jpn. 79:702
Ali A., Nain A.K., Lal B., Chand D. (2004). Int. J. Thermophys. 25:1835
Dean J.A. (1956). Lange’s Handbook of Chemistry. McGraw Hill, New York
J. Feeney and L. H. Sutcliffe, J. Chem. Soc. 1123 (1962).
Marcus Y. (1977). Introduction to Liquid State Chemistry. Wiley Interscience, New York
Eads C.D. (2000). J. Phys. Chem. B 104:6653
Saleh M.A., Alauddin M., Begum S. (2001). Phys. Chem. Liq. 39:453
Nikam P.S., Hasan M., Patil V.U. (2000). Ind. J. Pure Appl. Phys. 38:693
Vogel A.I. (1989). Text Book of Practical Organic Chemistry, 5th Ed. Longman Green, London
Riddick J.A., Bunger W.B., Sakano T. (1986). Organic Solvents: Physical Properties and Methods of Purification, 4th Ed. Wiley-Interscience, New York
Stokes R.H., Mills R. (1965). Viscosity of Electrolytes and Related Properties. Pergamon Press, New York
D. R. Lide and H. P. R. Frederike, eds., CRC Handbook of Chemistry and Physics, 81st Ed. (CRC Press, Boca Raton, Florida, 2001).
Grenner A., Clauck M., Kramer M., Schmelzer J. (2006). J. Chem. Eng. Data 51:176
Deshpande D.D., Bhatgadde L.G. (1968). J. Phys. Chem. 72:261
Jiménez E., Franzo C., Segade L., Legido J.L., Paz Andrade M.I. (1998). J. Solution Chem. 27:569
Ortega J. (1982). J. Chem. Eng. Data 27:312
Jiménez A.E., Cabanas M., Segade L., Garcia-Garabal S., Casas H. (2001). Fluid Phase Equilib. 180:151
Sandhu J.S., Sharma A.K., Wadi R.K. (1986). J. Chem. Eng. Data 31:152
Zeilkiewicz A.J. (1995). J. Chem. Thermodyn. 27:225
Morrone S.R., Francesconi A.Z. (1996). J. Chem. Thermodyn. 28:935
Venkatesulu D., Venkatesu P., Rao M.V.P. (1996). J. Chem. Eng. Data 41:819
Ritzooulis G. (1989). Can. J. Chem. 67:1105
Anson A., Garriga R., Martinez S., Perez P., Gracia M. (2005). J. Chem. Eng. Data 50:677
Giner B., Artigas H., Carrion A., Lafuente C., Royo F.M. (2003). J. Mol. Liq. 108:303
TRC Thermodynamic Tables - Non-hydrocarbons (Thermodynamics Research Center, Texas A&M University System, College Station, Texas, 1993).
TRC Thermodynamic Tables - Non-hydrocarbons (Thermodynamics Research Center, Texas A&M University System, College Station, Texas, 1966).
TRC Thermodynamic Tables - Non-hydrocarbons (Thermodynamics Research Center, Texas A&M University System, College Station, Texas, 1984).
Artigas H., Rodriguez V., Martin S., Cea P., Lopez M.C. (2002). Int. J. Thermophys. 23:1455
Nikam P.S., Shirsat L.N., Hasan M. (1998). J. Chem. Eng. Data 44:732
Weng W. (1999). J. Chem. Eng. Data 44:788
Redlich O., Kister A.T. (1948). Ind. Eng. Chem. 40:345
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nain, A.K. Densities and Volumetric Properties of Binary Mixtures of Aniline with 1-Propanol, 2-Propanol, 2-Methyl-1-Propanol, and 2-Methyl-2-Propanol at Temperatures from 293.15 to 318.15 K. Int J Thermophys 28, 1228–1244 (2007). https://doi.org/10.1007/s10765-007-0204-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-007-0204-0