Abstract
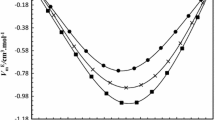
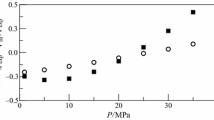
Densities ρ of the 1-butanol + chloroform + benzene ternary mixture and the 1-butanol + chloroform and 1-butanol + benzene binaries have been measured at six temperatures (288.15, 293.15, 298.15, 303.15, 308.15, and 313.15) K and atmospheric pressure, using an oscillating U-tube densimeter. From these densities, excess molar volumes (V E) were calculated and fitted to the Redlich–Kister equation for all binary mixtures and to the Nagata and Tamura equation for the ternary system. The Radojković et al. equation has been used to predict excess molar volumes of the ternary mixtures. Also, V E data of the binary systems were correlated by the van der Waals (vdW1) and Twu–Coon–Bluck–Tilton (TCBT) mixing rules coupled with the Peng–Robinson–Stryjek–Vera (PRSV) equation of state. The prediction and correlation of V E data for the ternary system were performed by the same models.
Similar content being viewed by others
References
Grgurić I.R., Šerbanović S.P., Kijevčanin M.Lj., Tasić A.Ž., Djordjević B.D. (2004). Thermochim. Acta 412: 25
Kijevčanin M.Lj., Djordjević B.D., Šerbanović S.P., Grgurić I.R., Tasić A.Ž. (2004). Phys. Chem. Liq. 42: 147
Šerbanović S.P., Kijevčanin M.Lj., Radović I.R., Djordjević B.D. (2006). Fluid Phase Equilib. 239: 69
Kijevčanin M.Lj., Šerbanović S.P., Radović I.R., Djordjević B.D., Tasić A.Ž. (2007). Fluid Phase Equilib. 251: 78
Kijevčanin M.Lj., Djuriš M.M., Radović I.R., Djordjević B.D., Šerbanović S.P. (2007). J. Chem. Eng. Data 52: 1136
Kijevčanin M.Lj., Purić I.M., Radović I.R., Djordjević B.D., Šerbanović S.P. (2007). J. Chem. Eng. Data 52: 2067
Lang Z.H., Jun H.S. (1996). Phys. Chem. Liq. 31: 49
Munk P., Qin A., Hoffman D. (1993). Collect. Czech. Chem. Commun. 58: 2612
Yu C.H., Tsai F.N. (1994). J. Chem. Eng. Data 39: 441
Ali A., Nain A.K., Lal B., Chand D. (2004). Int. J. Thermophys. 25: 1835
Bhardway U., Maken S., Singh K.C. (1996). J. Chem. Thermodyn. 28: 1173
Redlich O., Kister A. (1948). Ind. Eng. Chem. 40: 345
Nagata I., Tamura K. (1990). J. Chem. Thermodyn. 22, 279
Radojković N., Tasić A., Grozdanić D., Djordjević B., Malić D. (1977). J. Chem. Thermodyn. 9, 349
Adachi Y., Sugie H. (1986). Fluid Phase Equilib. 23, 103
Twu C.H., Coon J.E., Bluck D., Tilton B. (1999). Fluid Phase Equilib. 158–160: 271
Riddick J.W., Bunger W.B., Sakano T.K. (1986). Techniques of Chemistry. Wiley, New York
Francesconi R., Lunelli B., Comelli F. (1996). J. Chem. Eng. Data 41, 310
Radojković N., Tasić A., Djordjević B., Grozdanić D. (1976). J. Chem. Thermodyn. 8: 1111
Tasić A.Ž., Grozdanić D.K., Djordjević B.D., Šerbanović S.P., Radojković N. (1995). J. Chem. Eng. Data 40: 586
Bevington P.R., Robinson D.K. (1994). Data Reduction and Error Analysis for the Physical Sciences. McGraw-Hill, Singapore
Assarsson P., Eirich F.R. (1968). J. Phys. Chem. 72: 2710
Stryjek R., Vera J.H. (1986). Can. J. Chem. Eng. 64, 323
Renon H., Prausnitz J.M. (1968). AIChE J. 14, 135
Marquardt D.W. (1963). J. Soc. Ind. Appl. Math. 2: 431
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smiljanić, J.D., Kijevčanin, M.L., Djordjević, B.D. et al. Temperature Dependence of Densities and Excess Molar Volumes of the Ternary Mixture (1-Butanol + Chloroform + Benzene) and its Binary Constituents (1-Butanol + Chloroform and 1-Butanol + Benzene). Int J Thermophys 29, 586–609 (2008). https://doi.org/10.1007/s10765-008-0390-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-008-0390-4