Abstract
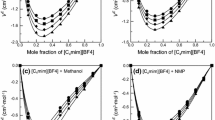
Apparent molar volumes, \(V_\phi\) , and compressibilities, \(\kappa _\phi\) , of 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIm][BF4]) have been determined from precise density and speed-of-sound measurements in organic solvents, methanol (MeOH), acetonitrile (MeCN), tetrahydrofuran (THF), N,N-dimethylacetamide (DMA), and dimethylsulfoxide (DMSO) in the dilute region of the ionic liquid. Corresponding values at infinite dilution are estimated by the Redlich–Mayer and Pitzer equations. The results have been interpreted by the interaction of the [BMIm][BF4] in the organic solvents. Results show that the structure and dielectric constant of the organic solvents play an important role for the ion–solvent interactions in these mixtures. It was found that the strength of interaction between [BMIm][BF4] with the studied organic solvents has the order DMSO > DMA > MeOH > MeCN > THF.
Similar content being viewed by others
References
Welton T. (1999). Chem. Rev. 99: 2071
Earle M.J., Seddon K.R. (2000). Pure Appl. Chem. 72: 13910
Dupont J., R.F. de Souza, P.Suarez A.Z. (2002). Chem. Rev. 102: 3667
Rodgers R.D., Seddon K.R. (2003). Science 302: 792
Zafarani-Moattar M.T., Shekaari H. (2005). J. Chem. Eng. Data 5: 1694
Zafarani-Moattar M.T., Shekaari H. (2005). J. Chem. Thermodyn. 37: 1029
Zafarani-Moattar M.T., Shekaari H. (2006). J. Chem. Thermodyn. 38: 624
Zafarani-Moattar M.T., Shekaari H. (2006). J. Chem. Thermodyn. 38: 1377
Shekaari H., Zafarani-Moattar M.T. (2007). Fluid Phase Equilib. 254: 198
Malham I.B., Letellier P., Mayaffre A., Turmine M. (2007). J. Chem. Thermodyn. 39: 1132
Zhou Q., Wang L.-S., Chen H.-P. (2006). J. Chem. Eng. Data 51: 905
de Azevedo R.G., Esperança J.M.S.S., Najdanovic-Visak V., Visak Z.P., Guedes H.J.R., da Ponte M.N., Rebelo L.P.N. (2005). J. Chem. Eng. Data 50: 997
Fredlake C.P., Crosthwaite J.M., Hert D.G., Aki S.N.V.K., Brennecke J.F. (2004). J. Chem. Eng. Data 49: 954
Zhang L.-Z., Deng D.-S., Han J.-Z., Ji D.-X., Ji J.-B. (2007). J. Chem. Eng. Data 52, 199
Lei Z., Arlt W., Wasserscheid P. (2006). Fluid Phase Equilib. 241, 290
Trindade J.R., Visak Z.P., Blesic M., Marrucho I.M., Coutinho J.A.P., Lopes J.N.C., Rebelo L.P.N. (2007). J. Chem. Eng. Data 52, 80
Makowska A., Siporska A., Szydłowski J. (2006). J. Phys. Chem. B 110: 17195
Kim K.-S., Park S.-Y., Choi S., Lee H. (2004). J. Chem. Eng. Data 49: 1550
Zhou Q., Wang L.-S. (2006). J. Chem. Eng. Data 51: 1698
Zhou Q., Wang L.-S., Wu L.-S., Li M-Y (2007). J. Chem. Eng. Data 52, 131
Shang H.-T., Wu J.-S., Zhou Q., Wang L-S. (2006). J. Chem. Eng. Data 51: 1286
Yokozeki A., Shiflett M.B. (2007). Ind. Eng. Chem. Res. 46: 1605
Wang J., Tian Y., Zhao Y., Zhuo K. (2003). Green Chem. 5: 618
Katayanagi H., Nishikawa K., Shimozaki H., Miki K., Chiba N., Westh P., Koga Y. (2004). J. Phys. Chem. B 108: 19451
Bowers J., Butts C.P., Martin P.J., Vergara-Gutierrez M.C., Heenan R.K. (2004). Langmuir 20: 2191
Comminges C., Rarhdadi R., Laurent M., Troupel M. (2006). J. Chem. Eng. Data 51: 680
Malham I.B., Letellier P., Turmine M. (2006). J. Phys. Chem. B 110: 14212
Sarkar A., Pandey S. (2006). J. Chem. Eng. Data 51: 2051
Marcus Y., Hefter G. (2004). Chem. Rev. 104: 3405
Redlich O., Mayer D.M. (1964). Chem. Rev. 64, 221
Rodgers P.S.Z., Pitzer K.S. (1982). J. Phys. Chem. Ref. Data 11, 15
Roy M.N., Dey R., Jha A. (2001). J. Chem. Eng. Data 46: 1327
Zhao Y., Wang J., Lu H., Lin R. (2004). J. Chem. Thermodyn. 36, 1
Brocos P., Pineiro N., Bravo R., Amigo A., Roux A.H., Roux-Desgranges G. (2002). J. Chem. Eng. Data 47, 351
Afanasyef V., Zyatkova L. (1996). J. Chem. Eng. Data 41: 1315
Saha N., Das B., Hazra D. (1995). J. Chem. Eng. Data 40: 1264
Prolongo M.G., Masegosa R.M., Fuentes I.H., Horta A. (1984). J. Phys. Chem. 88: 2163
Krakowiak J., Bobicz D., Grzybkowski W. (2000). J. Mol. Liq. 88: 197
Aminabhavi T.M., Bindu G. (1995). J. Chem. Eng. Data 40: 856
Marcus Y., Hefter G. (1999). J. Solution Chem. 28: 575
Lankford J.I., Holladay W.T., Criss C. (1984). J. Solution Chem. 13, 699
Resa J.M., Gonzalez C., de Landaluce S.O., Lanz J. (2002). J. Chem. Thermodyn. 34: 1013
Krakowiak J., Koziel H., Grzybkowski W. (2004). J. Mol. Liq. 112, 171
Scharlin P., Steinby K. (2003). J. Chem. Thermodyn. 35, 279
Sekhar G.C., Rao M.V.P., Prasad D.H.L., Kumar Y.V.L.R. (2003). Thermochim. Acta 402, 99
Syal V.K., Chauhan S., Gautam R. (1998). Ultrasonics 36, 619
Lankford J.I., Criss C. (1987). J. Solution Chem. 16, 753
Fredlake C.P., Crosthwaite J.M., Hert D.G., Aki S.N.V.K., Brennecke J.F. (2004). J. Chem. Eng. Data 49, 954
Hanke C.G., Atamas N.A., Lyndeln-Bell R.M. (2002). Green Chem. 4, 107
Dey N.C., Bhuyan J., Haque I. (2003). J. Solution Chem. 32, 547
Ananthaswamyt J., Atkinson G. (1984). J. Chem. Eng. Data 29, 81
Das D., Das B., Hazra D. (2004). J. Mol. Liq. 111, 15
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shekaari, H., Zafarani-Moattar, M.T. Volumetric Properties of the Ionic Liquid, 1-Butyl-3-methylimidazolium Tetrafluoroborate, in Organic Solvents at T = 298.15K. Int J Thermophys 29, 534–545 (2008). https://doi.org/10.1007/s10765-008-0395-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-008-0395-z