Abstract
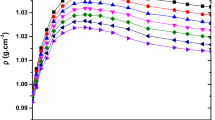
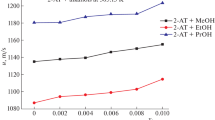
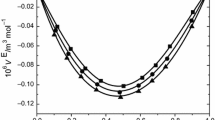
Densities and viscosities were measured for binary mixtures of isoamyl alcohol with 2-methoxyethanol, 2-ethoxyethanol, and 2-butoxyethanol over the entire range of composition at 303.15 K, 313.15 K, and 323.15K and ultrasonic speeds and refractive indices at 303.15 K under atmospheric pressure. From the experimental values of density, viscosity, ultrasonic speed, and refractive index, the values of excess molar volume (V E), viscosity deviations (Δη), deviations in isentropic compressibility (ΔK S ), and excess molar refraction (ΔR) have been calculated. The excess or deviation properties were found to be either negative or positive, depending on the molecular interactions and the nature of liquid mixtures.
Similar content being viewed by others
References
Reid C.R., Poling B.E.: The Properties of Gases and Liquids. McGraw Hill, New York (1998) Chap. 1
Perez E., Cardoso M., Mainar A.M., Pardo J.I., Urieta J.S.: J. Chem. Eng. Data 48, 1306 (2003)
Nayak J.N., Aminabhavi T.M., Aralaguppi M.I.: J. Chem. Eng. Data 48, 1152 (2003)
Sastry N.V., Patel S.R.: Int. J. Thermophys. 21, 1153 (2000)
Roy M.N., Choudhury A., Sinha A.: J. Teach. Res. Chem 11, 12 (2004)
Hazra D.K., Roy M.N., Das B.: Indian J. Chem. Technol. 1, 93 (1994)
Roy M.N., Jha A., Dey R.: J. Chem. Eng. Data 46, 1327 (2001)
Roy M.N., Sinha A., Sinha B.: J. Sol. Chem. 34, 1319 (2005)
Nikam P.S., Shirsat L.N., Hasan M.: J. Indian Chem. Soc. 77, 244 (2000)
Ujjan B.K., Apoorva P.H, Arun B.S., Mehdi H.: J. Chem. Eng. Data 51, 60 (2006)
Aminabhabi T.N., Gopalkrisna B.: J. Chem. Eng. Data 39, 529 (1994)
Singh M.: J. Ind. Chem. Soc. 79, 659 (2002)
Glasstone S., Laidler K.J., Eyring H.: The Theory of Rate Processes: The Kinetics of Chemical Reactions, Viscosity, Diffusion and Electrochemical Phenomena. McGraw-Hill, New York (1941)
Brocos P., Pineiro A., Bravo R., Amigo A.: Phys. Chem. Chem. Phys. 5, 550 (2003)
Pineiro A., Brocos P., Amigo A., Pintos M., Bravo R.: Phys. Chem. Liq. 38, 251 (2000)
Gill D.S., Cheema T.S.: Z. Phys. Chem. (N.F.) 134, 205 (1983)
Marcus Y.: Ion Solvation. Wiley, New York (1985)
Minkin, V., Osipov, O., Zhdanov, Y. (eds): Dipole Moments in Organic Chemistry. Plenum Press, New York, London (1970)
Roy M.N., Sinha A.: Fluid Phase Equilib. 243, 133 (2006)
Venkatesulu D., Venkatesu P., Rao M.V.P.: J. Chem. Eng. Data 42, 365 (1997)
Mozo I., de la Fuente I.G., Gonzalez J.A., Cobos J.C., Riesco N.: J. Chem. Eng. Data 53, 1404 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roy, M.N., Sah, R.S. & Pradhan, P. Densities, Viscosities, Sound Speeds, Refractive Indices, and Excess Properties of Binary Mixtures of Isoamyl Alcohol with Some Alkoxyethanols. Int J Thermophys 31, 316–326 (2010). https://doi.org/10.1007/s10765-010-0719-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-010-0719-7