Abstract
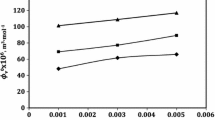
Speeds of sound of (l-alanine/l-glutamine/glycylglycine \(\,+\, 0.512\, {\mathrm{mol}}\cdot {\mathrm{kg}}^{-1}\) aqueous \({\mathrm{KNO}}_{3}/0.512\, {\mathrm{mol}}\cdot {\mathrm{kg}}^{-1}\) aqueous \({\mathrm{K}}_{2}{\mathrm{SO}}_{4}\)) systems have been measured for several molal concentrations of amino acid/peptide at different temperatures: \(T\)= (298.15 to 323.15) K. Using the speed-of-sound and density data, the parameters, partial molar isentropic compressibilities \(\phi _{\kappa }^{0}\) and transfer partial molar isentropic compressibilities \(\Delta _{\mathrm{tr}} \phi _{\kappa }^{0}\), have been computed. The trends of variation of \(\phi _{\kappa }^{0}\) and \(\Delta _{\mathrm{tr}} \phi _{\kappa }^{0}\) with changes in molal concentration of the solute and temperature have been discussed in terms of zwitterion–ion, zwitterion–water dipole, ion–water dipole, and ion–ion interactions operative in the systems.
Similar content being viewed by others
References
M. Hoefling, K.E. Gottschalk, Computational Biophysics to System Biology, in Proceedings of the NIC Workshop, NIC Series, vol. 40, 2008, p. 231
B.W. Matthews, Encyclopedia of Life Science (Nature Publishing Group, London, 2001)
R. Sadeghi, B. Goodarzi, J. Mol. Liq. 141, 62 (2008)
G. Lin, P. Bian, R. Lin, J. Chem. Thermodyn. 38, 144 (2006)
A. Pal, S. Kumar, J. Mol. Liq. 121, 148 (2005)
R. Badarayani, A. Kumar, J. Chem. Thermodyn. 35, 897 (2003)
K. Rajagopal, S.E. Gladson, J. Chem. Thermodyn. 43, 852 (2011)
H. Rodriguez, A. Soto, A. Arce, M.K. Khoshkbarchi, J. Solution Chem. 32, 53 (2003)
A.N. Kannappan, R. Palani, Indian J. Chem. 46A, 54 (2007)
Q. Yuan, Z. Li, B. Wang, J. Chem. Thermodyn. 38, 20 (2006)
T.S. Banipal, J. Kaur, P.K. Banipal, K. Singh, J. Chem. Eng. Data 53, 1803 (2008)
Riyazuddeen, T. Altamash, Thermochim. Acta 501, 72 (2010)
Riyazuddeen, I. Khan, Int. J. Thermophys. 30, 475 (2009)
Riyazuddeen, G.K. Bansal, Thermochim. Acta 445, 40 (2006)
Riyazuddeen, R. Basharat, J. Chem. Thermodyn. 38, 1684 (2006)
Riyazuddeen, I. Khan, Thermochim. Acta 483, 45 (2009)
Riyazuddeen, U. Gazal, J. Chem. Eng. Data 57, 7 (2012)
M.K. Khoshkbarchi, J.H. Vera, Ind. Eng. Chem. Res. 35, 87 (1996)
M. Natarajan, R.K. Wadi, H.C. Gaur, J. Chem. Eng. Data 35, 87 (1990)
T.V. Chalikian, A.P. Sarvazyan, T. Funk, C.A. Cain, K.J. Breaslauer, J. Phys. Chem. 98, 321 (1994)
Riyazuddeen, U. Gazal, J. Chem. Eng. Data 57, 1468 (2012)
Landolt-Bornstein, Neue Serie, Molekularakustik Temperaturskala von 100 (1990), p. 195
F.T. Gucker, Chem. Rev. 13, 111 (1933)
P. Debye, E. Hückle, Z. Phys. 24, 185 (1923)
R. Sadeghi, A. Gholamireza, J. Chem. Thermodyn. 43, 200 (2011)
F.J. Millero, A.L. Surdo, C. Shin, J. Phys. Chem. 82, 784 (1978)
A. Soto, A. Arce, M.K. Khoshkbarchi, J. Solution Chem. 33, 11 (2004)
G.R. Hedwig, Pure Appl. Chem. 66, 387 (1994)
B.E. Conway, R.E. Verall, J. Phys. Chem. 70, 3952 (1966)
M. Kikuchi, M. Sakurai, K. Nitta, J. Chem. Eng. Data 40, 935 (1995)
T.S. Banipal, P. Kapoor, J. Indian Chem. Soc. 76, 431 (1999)
D.P. Kharakoz, J. Phys. Chem. 95, 5634 (1991)
R.K. Wadi, P. Ramasami, J. Chem. Soc., Faraday Trans. 93, 243 (1997)
Y. Yasuda, N. Tochio, M. Sakurai, K. Nitta, J. Chem. Eng. Data 43, 205 (1998)
P. Ramasami, R. Kakkar, J. Chem. Thermodyn. 38, 144 (2006)
T.S. Banipal, G. Sehgal, Thermochim. Acta 262, 175 (1995)
Acknowledgments
The authors are thankful to the Chairman Department of Chemistry, A.M.U. Aligarh for providing the necessary facility for the compilation of this study. Financial support from the UGC-SAP (DRS-I) scheme is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Riyazuddeen, Gazal, U. Transfer Partial Molar Isentropic Compressibilities of (l-Alanine/l-Glutamine/Glycylglycine) from Water to 0.512 \({\mathrm{mol}}\!\cdot \!{\mathrm{kg}}^{-1}\) Aqueous \({\mathrm{KNO}}_{3}/0.512\, {\mathrm{mol}}\!\cdot \! {\mathrm{kg}}^{-1}\) Aqueous \({\mathrm{K}}_{2}{\mathrm{SO}}_{4}\) Solutions Between 298.15 K and 323.15 K. Int J Thermophys 34, 424–433 (2013). https://doi.org/10.1007/s10765-013-1432-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-013-1432-0