Abstract
Significance
Our results show that asthmatic patients tend to have more severe KC and thus close monitoring for disease progression would be advised, and appropriate treatment strategies may be actioned stabilise the condition that may reduce the need for future corneal transplantation.
Purpose
To explore a wide range of risk factors associated with the severity of keratoconus (KC).
Methods
A cross-sectional study of KC patients was undertaken in Melbourne, Australia. A questionnaire addressing age, gender, educational background, ocular and medical history, smoking and alcohol consumption, and physical examination comprising anthropometric measurements was collected; eye examination was undertaken. The associations between a range of risk factors and the severity of KC were determined using univariate and multivariable linear regression analyses.
Results
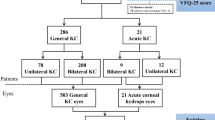
A total of 260 KC subjects were included in this study. Mean age of subject was 35.5 (SD = 14.8) years and the majority of the subjects were European 171 (68.2%). Initial univariate regression analysis identified the following risk factors at the p < 0.1 level with KC: higher body mass index, smoking cigarettes, diabetes, rheumatoid arthritis and asthma were associated with increased severity of KC, whereas eczema was associated with less severe KC. Following multivariable regression analysis, only asthma remained as a significant risk factor associated with 2.2 diopters (D) steeper average mean keratometry compared to KC subjects having no asthma [p = 0.03; β = 2.18; 95% confidence intervals: 1.22, 4.14].
Conclusion
Our study describes the comprehensive assessment of all the known risk factors in a large KC cohort recruited in Australia. Our study has reported asthma as the only risk factor found to be significantly associated with the severity of KC. The results of this study allow us to better understand the aetiology of KC and such knowledge could be useful in instigate systemic management of patients to slow or prevent KC.
Similar content being viewed by others
References
Rabinowitz YS (1998) Keratoconus. Surv Ophthalmol 42:297–319
Godefrooij DA, Mangen MJ, Chan E et al (2017) Cost-effectiveness analysis of corneal collagen crosslinking for progressive keratoconus. Ophthalmology 124:1485–1495
Williams KA, Lowe M, Bartlett C et al (2008) Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation 86:1720–1724
Davidson AE, Hayes S, Hardcastle AJ et al (2014) The pathogenesis of keratoconus. Eye (Lond) 28:189–195
Balasubramanian SA, Pye DC, Willcox MD (2013) Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: relevance in keratoconus. Clin Exp Optom 96:214–218
Galvis V, Tello A, Carreno NI et al (2017) Risk factors for keratoconus: atopy and eye rubbing. Cornea 36:e1
Barr JT, Wilson BS, Gordon MO et al (2006) Estimation of the incidence and factors predictive of corneal scarring in the collaborative longitudinal evaluation of keratoconus (CLEK) study. Cornea 25:16–25
Szczotka LB, Barr JT, Zadnik K (2001) A summary of the findings from the collaborative longitudinal evaluation of keratoconus (CLEK) study. CLEK Study Group Optom 72:574–584
Wagner H, Barr JT, Zadnik K (2007) Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: methods and findings to date. Cont Lens Anterior Eye 30:223–232
Zadnik K, Barr JT, Edrington TB et al (1998) Baseline findings in the collaborative longitudinal evaluation of keratoconus (CLEK) study. Invest Ophthalmol Vis Sci 39:2537–2546
Zadnik K, Barr JT, Edrington TB et al (2000) Corneal scarring and vision in keratoconus: a baseline report from the collaborative longitudinal evaluation of keratoconus (CLEK) study. Cornea 19:804–812
Zadnik K, Barr JT, Gordon MO et al (1996) Biomicroscopic signs and disease severity in keratoconus. collaborative longitudinal evaluation of keratoconus (CLEK) study group. Cornea 15:139–146
Weed KH, Macewen CJ, Giles T et al (2008) The Dundee University Scottish Keratoconus study: demographics, corneal signs, associated diseases, and eye rubbing. Eye (Lond) 22:534–541
Agrawal VB (2011) Characteristics of keratoconus patients at a tertiary eye center in India. J Ophthalmic Vis Res 6:87–91
Woodward MA, Blachley TS, Stein JD (2016) The association between sociodemographic factors, common systemic diseases, and keratoconus: an analysis of a nationwide heath care claims database. Ophthalmology 123(457–465):e452
Harrison RJ, Klouda PT, Easty DL et al (1989) Association between keratoconus and atopy. Br J Ophthalmol 73:816–822
Khor WB, Wei RH, Lim L et al (2011) Keratoconus in Asians: demographics, clinical characteristics and visual function in a hospital-based population. Clin Experiment Ophthalmol 39:299–307
Nemet AY, Vinker S, Bahar I et al (2010) The association of keratoconus with immune disorders. Cornea 29:1261–1264
Owens H, Gamble G (2003) A profile of keratoconus in New Zealand. Cornea 22:122–125
Burdon KP, Coster DJ, Charlesworth JC et al (2008) Apparent autosomal dominant keratoconus in a large Australian pedigree accounted for by digenic inheritance of two novel loci. Hum Genet 124:379–386
Burdon KP, Macgregor S, Bykhovskaya Y et al (2011) Association of polymorphisms in the hepatocyte growth factor gene promoter with keratoconus. Invest Ophthalmol Vis Sci 52:8514–8519
Bykhovskaya Y, Li X, Epifantseva I et al (2012) Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Invest Ophthalmol Vis Sci 53:4152–4157
Li X, Rabinowitz YS, Tang YG et al (2006) Two-stage genome-wide linkage scan in keratoconus sib pair families. Invest Ophthalmol Vis Sci 47:3791–3795
Lu Y, Vitart V, Burdon KP et al (2013) Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat Genet 45:155–163
Shneor E, Millodot M, Blumberg S et al (2013) Characteristics of 244 patients with keratoconus seen in an optometric contact lens practice. Clin Exp Optom 96:219–224
Sahebjada S, Fenwick EK, Xie J et al (2014) Impact of keratoconus in the better eye and the worse eye on vision-related quality of life. Invest Ophthalmol Vis Sci 55:412–416
Sahebjada S, Schache M, Richardson AJ et al (2014) Association of the hepatocyte growth factor gene with keratoconus in an Australian population. Plos One 9:e84067
Sahebjada S, Schache M, Richardson AJ et al (2013) Evaluating the association between keratoconus and the corneal thickness genes in an independent Australian population. Invest Ophthalmol Vis Sci 54:8224–8228
Sabiston DW (1966) The association of keratoconus, dermatitis and asthma. Trans Ophthalmol Soc New Zealand 18:66–71
Merdler I, Hassidim A, Sorkin N et al (2015) Keratoconus and allergic diseases among Israeli adolescents between 2005 and 2013. Cornea 34:525–529
Bak-Nielsen S, Ramlau-Hansen CH, Ivarsen A et al (2018) A nationwide population-based study of social demographic factors, associated diseases and mortality of keratoconus patients in Denmark from 1977 to 2015. Acta Ophthalmol. https://doi.org/10.1111/aos.13961
To T, Stanojevic S, Moores G et al (2012) Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Pub Health 12:204
Cookson WO, Moffatt MF (2000) Genetics of asthma and allergic disease. Hum Mol Genet 9:2359–2364
Ellwood P, Asher MI, Billo NE et al (2017) The global asthma network rationale and methods for phase i global surveillance: prevalence, severity, management and risk factors. Eur Respir J. https://doi.org/10.1183/13993003.01605-2016
Sabti S, Tappeiner C, Frueh BE (2015) Corneal cross-linking in a 4-year-old child with keratoconus and down syndrome. Cornea 34:1157–1160
Kankariya VP, Kymionis GD, Diakonis VF et al (2013) Management of pediatric keratoconus - evolving role of corneal collagen cross-linking: an update. Indian J Ophthalmol 61:435–440
Ferreira MA, Matheson MC, Duffy DL et al (2011) Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. Lancet 378:1006–1014
Jensen LJ, Kuhn M, Stark M et al (2009) STRING 8–a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res 37:D412-416
Shetty R, Sharma A, Pahuja N et al (2017) Oxidative stress induces dysregulated autophagy in corneal epithelium of keratoconus patients. Plos One 12:e0184628
Li YR, Li J, Zhao SD et al (2015) Meta-analysis of shared genetic architecture across ten pediatric autoimmune diseases. Nat Med 21:1018–1027
An-Nakhli FR (2015) Association between diabetes and keratoconus: a case-control study. Cornea 34:e10
Knox Cartwright NE, Tole DM (2011) Diabetes and keratoconus. Ophthalmology. https://doi.org/10.1016/j.ophtha.2010.08.022
Kosker M, Rapuano CJ (2015) Association between diabetes and keratoconus: a case-control study. Cornea 34:e17
Kosker M, Suri K, Hammersmith KM et al (2014) Another look at the association between diabetes and keratoconus. Cornea 33:774–779
Kuo IC, Broman A, Pirouzmanesh A et al (2006) Is there an association between diabetes and keratoconus? Ophthalmology 113:184–190
Naderan M, Naderan M, Rezagholizadeh F et al (2014) Association between diabetes and keratoconus: a case-control study. Cornea 33:1271–1273
Prakash G, Sharma N, Titiyal JS (2007) Association between diabetes and keratoconus? Ophthalmology. https://doi.org/10.1016/j.ophtha.2006.10.062
Seiler T, Huhle S, Spoerl E et al (2000) Manifest diabetes and keratoconus: a retrospective case-control study. Graefes Arch Clin Exp Ophthalmol 238:822–825
Goldich Y, Barkana Y, Gerber Y et al (2009) Effect of diabetes mellitus on biomechanical parameters of the cornea. J Cataract Refract Surg 35:715–719
Shah S, Laiquzzaman M, Bhojwani R et al (2007) Assessment of the biomechanical properties of the cornea with the ocular response analyzer in normal and keratoconic eyes. Invest Ophthalmol Vis Sci 48:3026–3031
Touboul D, Roberts C, Kerautret J et al (2008) Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J Cataract Refract Surg 34:616–622
Greene PR, Medina A (2016) The progression of nearwork myopia. Optom Open Access. https://doi.org/10.4172/2476-2075.1000120
Acknowledgements
The authors wish to thank participants from the Keratoconus study who made this work possible. The authors would also like to thank the Eye surgery associates, Lindsay and associates and Keratoconus Australia, Mr Tony Ngo for their assistance with recruitment. A preliminary report on some of these data was presented at the Asia cornea society meeting, Taipei, Taiwan, Dec, 2014.
Funding
This study was supported by the Australian National Health and Medical Research Council (NHMRC) project ideas grant APP1187763 and senior research fellowship (1138585 to PNB), Lions Eye Donation Service (SS), Angior Family Foundation (SS) and Perpetual Impact Philanthropy grant (SS). The Centre for Eye Research Australia (CERA) receives operational infrastructure support from the Victorian government. The sponsor or funding organizations had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Royal victorian eye and ear hospital human research and ethics committee (Project # 10/954H) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sahebjada, S., Chan, E., Xie, J. et al. Risk factors and association with severity of keratoconus: the Australian study of Keratoconus. Int Ophthalmol 41, 891–899 (2021). https://doi.org/10.1007/s10792-020-01644-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01644-6