Abstract
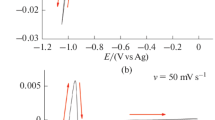
The Electroseparation of zinc(II) from a solution of uranium(III) generated by the reduction of uranium(IV) triflate (U(OTf)4) with zinc amalgam (Zn(Hg)) was studied to establish a convenient route to the precursors of organic uranium(III) compounds. Specifically, the electrode reactions of U(OTf)4 in N,N-dimethylformamide on mercury (Hg) and Zn(Hg) electrodes were probed by voltammetry and bulk electrolysis measurements. The voltammograms recorded on the former electrode showed three cathodic waves assigned to the reduction of a uranium(IV) solvate complex (− 1.48 V), the reduction of [U(OTf)]3+ (− 1.81 V), and uranium amalgamation (− 2.65 V). On the latter electrode, the first cathodic wave was masked, as it was located at the potential of zinc amalgamation. Bulk electrolysis experiments revealed that for both Hg and Zn(Hg), the working electrode potential featured a plateau around − 2.3 V, which was not observed in the corresponding voltammograms and was related to the degradation of uranium(III) based on spectroscopic observations. The Electroseparation of zinc(II) from the uranium(III) solution on Zn(Hg) was successfully (highest coulombic efficiency = 0.81, final zinc(II) separation ratio = 93.4%) carried out after the reduction of U(OTf)4 by Zn(Hg).
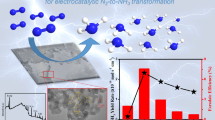
Graphical abstract







Similar content being viewed by others
References
Ephritikhine M (2006) The vitality of uranium molecular chemistry at the dawn of the XXIst century. Dalton Trans 21:2501–2516. https://doi.org/10.1039/b603463b
Korobkov I, Gorelsky S, Gambarotta S (2009) Reduced uranium complexes: synthetic and DFT study of the role of pi ligation in the stabilization of uranium species in a formal low-valent state. J Am Chem Soc 131(30):10406–10420. https://doi.org/10.1021/ja9002525
Antunes MA, Pereira LC, Santos IC, Mazzanti M, Marcalo J, Almeida M (2011) [U(Tp(Me2))2(bipy)]+: a cationic uranium(III) complex with single-molecule-magnet behavior. Inorg Chem 50(20):9915–9917. https://doi.org/10.1021/ic200705p
Mansell SM, Kaltsoyannis N, Arnold PL (2011) Small molecule activation by uranium tris(aryloxides): experimental and computational studies of binding of N2, coupling of CO, and deoxygenation insertion of CO2 under ambient conditions. J Am Chem Soc 133(23):9036–9051. https://doi.org/10.1021/ja2019492
Hohloch S, Garner ME, Parker BF, Arnold J (2017) New supporting ligands in actinide chemistry: tetramethyltetraazaannulene complexes with thorium and uranium. Dalton Trans 46(40):13768–13782. https://doi.org/10.1039/c7dt02682j
Boreen MA, Arnold J (2020) The synthesis and versatile reducing power of low-valent uranium complexes. Dalton Trans 49(43):15124–15138. https://doi.org/10.1039/d0dt03151h
Boreen MA, Gould CA, Booth CH, Hohloch S, Arnold J (2020) Structure and magnetism of a tetrahedral uranium(iii) beta-diketiminate complex. Dalton Trans 49(23):7938–7944. https://doi.org/10.1039/d0dt01599g
Riedhammer J, Aguilar-Calderon JR, Miehlich M, Halter DP, Munz D, Heinemann FW, Fortier S, Meyer K, Mindiola DJ (2020) Werner-type complexes of uranium(III) and (IV). Inorg Chem 59(4):2443–2449. https://doi.org/10.1021/acs.inorgchem.9b03229
Clark DL, Sattelberger AP, Bott SG, Vrtis RN (1989) Lewis base adducts of uranium triiodide: a new class of synthetically useful precursors for trivalent uranium chemistry. Inorg Chem 28(10):1771–1773. https://doi.org/10.1021/ic00309a004
Drożdżyński J (2005) Tervalent uranium compounds. Coord Chem Rev 249(21):2351–2373. https://doi.org/10.1016/j.ccr.2005.05.016
Carmichael CD, Jones NA, Arnold PL (2008) Low-valent uranium iodides: straightforward solution syntheses of UI3 and UI4 etherates. Inorg Chem 47(19):8577–8579. https://doi.org/10.1021/ic801138e
Fetrow TV, Grabow JP, Leddy J, Daly SR (2021) Convenient syntheses of trivalent uranium halide starting materials without uranium metal. Inorg Chem 60(11):7593–7601. https://doi.org/10.1021/acs.inorgchem.1c00598
Avens LR, Bott SG, Clark DL, Sattelberger AP, Watkin JG, Zwick BD (1994) A convenient entry into trivalent actinide chemistry: synthesis and characterization of AnI3(THF)4 and An[N(SiMe3)2]3 (An = U, Np, Pu). Inorg Chem 33(10):2248–2256. https://doi.org/10.1021/ic00088a030
Evans WJ, Kozimor SA, Ziller JW, Fagin AA, Bochkarev MN (2005) Facile syntheses of unsolvated UI3 and tetramethylcyclopentadienyl uranium halides. Inorg Chem 44(11):3993–4000. https://doi.org/10.1021/ic0482685
Monreal MJ, Thomson RK, Cantat T, Travia NE, Scott BL, Kiplinger JL (2011) UI4(1,4-dioxane)2, [UCl4(1,4-dioxane)]2, and UI3(1,4-dioxane)1.5: stable and versatile starting materials for low- and high-valent uranium chemistry. Organometallics 30(7):2031–2038. https://doi.org/10.1021/om200093q
Schleid T, Meyer G, Morss LR (1987) Facile synthesis of UCl4 and ThCl4, metallothermic reductions of UCl4 with alkali metals and crystal structure refinements of UCl3, UCl4 and Cs2UCl6. J Less Common Met 132(1):69–77. https://doi.org/10.1016/0022-5088(87)90175-5
La Pierre HS, Heinemann FW, Meyer K (2014) Well-defined molecular uranium(III) chloride complexes. Chem Commun (Camb) 50(30):3962–3964. https://doi.org/10.1039/c3cc49452g
Bullock JI, Storey AE (1977) Complexes of uranium(III) with bidentate amides. J Chem Soc Chem Commun. https://doi.org/10.1039/c39770000507
Moody DC, Odom JD (1979) The chemistry of trivalent uranium: the synthesis and reaction chemistry of the tetrahydrofuran adduct of uranium trichloride, UCl3(THF)x. J Inorg Nucl Chem 41(4):533–535. https://doi.org/10.1016/0022-1902(79)80439-X
Moody DC, Zozulin AJ, Salazar KV (1982) Improved synthesis of UCl3(THF)x and the preparation of 15-crown-5 derivatives of trivalent uranium. Inorg Chem 21(10):3856–3857. https://doi.org/10.1021/ic00140a055
Rossetto G, Zanella P, Paolucci G, De Paoli G (1982) The reduction of uranium tetracloride by lithium tetrahydroaluminate in tetrahydrofuran or dimethoxyethane. Inorg Chim Acta 61:39–42. https://doi.org/10.1016/S0020-1693(00)89116-1
Berthet J-C, Lance M, Nierlich M, Vigner J, Ephritikhine M (1991) Tricyclopentadienyluranium azide complexes. J Organomet Chem 420(2):C9–C11. https://doi.org/10.1016/0022-328x(91)80271-k
Hauchard D, Cassir M, Chivot J, Ephritikhine M (1991) Electrochemical study of uranium(IV) and uranium(IV) organometallic compounds in tetrahydrofuran by means of conventional microelectrodes and ultramicroelectrodes. J Electroanal Chem Interfacial Electrochem 313(1–2):227–241. https://doi.org/10.1016/0022-0728(91)85182-o
Mech A, Karbowiak M, Lis T, Drożdżyński J (2006) Monomeric, dimeric and polymeric structure of the uranium trichloride hydrates. Polyhedron 25(10):2083–2092. https://doi.org/10.1016/j.poly.2006.01.004
Daly SR, Girolami GS (2010) Synthesis, characterization, and structures of uranium(III) N,N-dimethylaminodiboranates. Inorg Chem 49(11):5157–5166. https://doi.org/10.1021/ic100290j
Hart FA, Tajik M (1983) Complexes of uranium(III) with cyclic polyethers and with aromatic diamines. Inorg Chim Acta 71:169–173. https://doi.org/10.1016/s0020-1693(00)83655-5
Kennedy JH (1960) Determination of uranium(IV) by reduction to uranium(III) in Jones reductor. Anal Chem 32(2):150–152. https://doi.org/10.1021/ac60158a002
Satô A (1967) Studies of the behavior of trivalent uranium in an aqueous solution. I. Its reduction and its stability in various acid solutions. Bull Chem Soc Jpn 40(9):2107–2110. https://doi.org/10.1246/bcsj.40.2107
Drocżdżyński J (1978) Absorption spectra of uranium(+ 3) in solution. J Inorg Nucl Chem 40(2):319–323. https://doi.org/10.1016/0022-1902(78)80131-6
Drożdżyński J (1984) Spectroscopic investigations of some uranium(III) complexes. J Mol Struct 114:449–454. https://doi.org/10.1016/0022-2860(84)87184-7
Yamamura T, Shirasaki K, Shiokawa Y (2006) Preparation of uranium(III) triflate in tetrahydrofuran by reduction of U(OTf)4 with zinc amalgam. J Phys Soc Jpn 75(Suppl):149–151. https://doi.org/10.1143/jpsjs.75s.149
Shen M, Li B, Li SZ, Yu JG (2011) Electrochemical removal of AlCl3 from LiCl-KCl Melts. Metall Mater Trans A 43(5):1662–1669. https://doi.org/10.1007/s11661-011-0982-7
Shen M, Li B, Yu J (2012) Investigation on electrochemical removal of CaCl2 from LiCl–KCl melts. Electrochim Acta 62:153–157. https://doi.org/10.1016/j.electacta.2011.12.007
Liu K, Liu Y-L, Chai Z-F, Shi W-Q (2021) Electroseparation of uranium from lanthanides (La, Ce, Pr, Nd and Sm) on liquid gallium electrode. Sep Purif Technol 265:118524. https://doi.org/10.1016/j.seppur.2021.118524
Chen GZ, Fray DJ (2001) Cathodic refining in molten salts: removal of oxygen, sulfur and selenium from static and flowing molten copper. J Appl Electrochem 31(2):155–164. https://doi.org/10.1023/A:1004175605236
Zhao X, Liu H, Li A, Shen Y, Qu J (2012) Bromate removal by electrochemical reduction at boron-doped diamond electrode. Electrochim Acta 62:181–184. https://doi.org/10.1016/j.electacta.2011.12.013
Giridhar P, Venkatesan KA, Subramaniam S, Srinivasan TG, Vasudeva Rao PR (2008) Extraction of uranium (VI) by 1.1 M tri-n-butylphosphate/ionic liquid and the feasibility of recovery by direct electrodeposition from organic phase. J Alloy Compd 448(1–2):104–108. https://doi.org/10.1016/j.jallcom.2007.03.115
Jagadeeswara Rao C, Venkatesan KA, Nagarajan K, Srinivasan TG, Vasudeva Rao PR (2011) Electrodeposition of metallic uranium at near ambient conditions from room temperature ionic liquid. J Nucl Mater 408(1):25–29. https://doi.org/10.1016/j.jnucmat.2010.10.022
Ohashi Y, Harada M, Asanuma N, Ikeda Y (2015) Feasibility studies on electrochemical recovery of uranium from solid wastes contaminated with uranium using 1-butyl-3-methylimidazorium chloride as an electrolyte. J Nucl Mater 464:119–127. https://doi.org/10.1016/j.jnucmat.2015.04.024
Krishna GM, Suneesh AS, Venkatesan KA, Antony MP (2016) Electrochemical interference of some fission products during the electrodeposition of uranium oxide from 1-butyl-3-methylimidazolium chloride ionic liquid. J Electroanal Chem 780:225–232. https://doi.org/10.1016/j.jelechem.2016.09.035
Sakamura Y, Omori T, Inoue T (2017) Application of electrochemical reduction to produce metal fuel material from actinide oxides. Nucl Technol 162(2):169–178. https://doi.org/10.13182/nt162-169
Herrmann SD, Li SX (2017) Separation and recovery of uranium metal from spent light water reactor fuel via electrolytic reduction and electrorefining. Nucl Technol 171(3):247–265. https://doi.org/10.13182/nt171-247
Behr B, Taraszewska J, Stroka J (1975) Kinetics of Zn2+ reduction at a Hg electrode from water-acetone and water-methanol mixtures. J Electroanal Chem Interfacial Electrochem 58(1):71–80. https://doi.org/10.1016/s0022-0728(75)80346-9
Andreu R, Sluyters-Rehbach M, Remijnse AG, Sluyters JH (1982) The mechanism of the reduction of Zn(II) from NaClO4 base electrolyte solutions at the DME. J Electroanal Chem Interfacial Electrochem 134(1):101–115. https://doi.org/10.1016/s0022-0728(82)85030-4
Taraszewska J, WaŁȩga A (1986) Medium effect: kinetics of Zn2+ reduction at the Hg electrode from water + dimethylformamide mixtures. J Electroanal Chem Interfacial Electrochem 200(1–2):261–277. https://doi.org/10.1016/0022-0728(86)90060-4
Michlmayr M, Gritzner G, Gutmann V (1966) Polarographic studies on uranium compounds in dimethylformamide and dimethylsulfoxide. Inorg Nuclear Chem Lett 2(8):227–231. https://doi.org/10.1016/0020-1650(66)80033-8
Shirasaki K, Yamamura T, Herai T, Shiokawa Y (2006) Electrodeposition of uranium in dimethyl sulfoxide and its inhibition by acetylacetone as studied by EQCM. J Alloy Compd 418(1–2):217–221. https://doi.org/10.1016/j.jallcom.2005.10.059
Shirasaki K, Yamamura T, Monden Y, Shiokawa Y (2006) Electrolytic reduction of U(IV) salts to U(III) in N,N-dimethylformamide: effects of electrode materials and counter anions. J Phys Soc Jpn 75(Suppl):152–154. https://doi.org/10.1143/jpsjs.75s.152
Izutsu K (2002) Redox Reactions in Non-Aqueous Solvents. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Acknowledgements
The author would like to express great acknowledgements to Prof. Tomoo Yamamura (Inst. for Integrated Radiation and Nuclear Science, Kyoto Univ.) and late associate Prof. Isamu Satoh for their kind encouragements and supports. This work was partly supported by JSPS KAKENHI [Grant No. 19760607].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shirasaki, K. Electroseparation of zinc(II) from uranium(III) prepared by reduction of uranium(IV) with zinc amalgam in dimethylformamide. J Appl Electrochem 52, 1101–1108 (2022). https://doi.org/10.1007/s10800-022-01698-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01698-7