Abstract
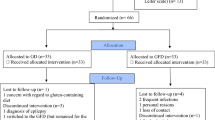
The use of alternative interventions, such as gluten-free and casein-free (GFCF) diets, is frequent due to limited therapies for Autism Spectrum Disorder (ASD). Our aims were to determine the influence of a GFCF diet on behavior disorders in children and adolescents diagnosed with ASD and the potential association with urinary beta-casomorphin concentrations. Thirty-seven patients were recruited for this crossover trial. Each patient consumed a normal diet (including gluten and casein) for 6 months and a GFCF diet for another 6 months. The order of the intervention (beginning with normal diet or with GFCF diet) was assigned randomly. Patients were evaluated at three time-points (at the beginning of the study, after normal diet and after GFCF diet). Questionnaires regarding behavior and autism and dietary adherence were completed and urinary beta-casomorphin concentrations were determined at each time-point. No significant behavioral changes and no association with urinary beta-casomorphin concentrations were found after GFCF diet. A 6-month GFCF diet do not induce significant changes in behavioral symptoms of autism and urinary beta-casomorphin concentrations. Further studies with a long follow-up period similar to ours and including placebo and blinding elements are needed to identify better those respondents to GFCF diets.



Similar content being viewed by others
References
Adams, J. B., Audhya, T., Geis, E., Gehn, E., Fimbres, V., Pollard, E. L., et al. (2018). Comprehensive nutritional and dietary intervention for autism spectrum disorder. A randomized, controlled 12-month trial. Nutrients,10, 369.
Aman, M. G., & Singh, N. N. (1986). Aberrant behavior checklist: Manual. East Aurora, NY: Slosson Educational Publications.
Amminger, G. P., Berger, G. E., Schäfer, M. R., Klier, C., Friedrich, M. H., & Feucht, M. (2007). Omega-3 fatty acids supplementation in children with autism: A double-blind randomized, placebo-controlled pilot study. Biological Psychiatry,61, 551–553.
Asperger, H. (1961). Psychopathology of children with celiac disease. Annals of Paediatrics,197, 346–351.
Barthélémy, C., Roux, S., Adrien, J. L., Hameury, L., Guérin, P., Garreau, B., et al. (1997). Validation of the Revised Behavior Summarized Evaluation Scale. Journal of Autism and Developmental Disorders,27, 139–153.
Bent, S., Bertoglio, K., Ashwood, P., Bostrom, A., & Hendren, R. L. (2011). A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder. Journal of Autism and Developmental Disorders,41, 545–554.
Bent, S., Hendren, R. L., Zandi, T., Law, K., Choi, J.-E., Widjaja, F., et al. (2014). Internet-based, randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism. Journal of American Academy of Child & Adolescent Psychiatry,53, 658–666.
Bertoglio, K., Jill James, S., Deprey, L., Brule, N., & Hendren, R. L. (2010). Pilot study of the effect of methyl B12 treatment on behavioral and biomarker measures in children with autism. Journal of Alternative and Complementary Medicine,16, 555–560.
Boukthir, S., Matoussi, N., Belhadj, A., Mammou, S., Dlala, S. B., Helayem, M., et al. (2010). Abnormal intestinal permeability in children with autism. Tunis Medicine,88, 685–686.
Cass, H., Gringras, P., March, J., McKendrick, I., O´Hare, A. E., Owen, L., et al. (2008). Absence of urinary opioid peptides in children with autism. Archives of Disease in Childhood,93, 745–750.
Catassi, C., Bai, J. C., Bonaz, B., Bouma, G., Calabró, A., Carroccio, A., et al. (2013). Non-Celiac Gluten sensitivity: The new frontier of gluten related disorders. Nutrients,5, 3839–3853.
Charman, T., Taylor, E., Drew, A., Cockerill, H., Brown, J., & Baird, G. (2015). Outcome at 7 years of children diagnosed with autism at age 2: Predictive validity of assessments conducted at 2 and 3 years of age and pattern of symptom change overtime. Journal of Child Psychology and Psychiatry and Allied Disciplines,46, 500–513.
Cieślińska, A., Sienkiewicz-Szłapka, E., Wasilewska, J., Fiedorowicz, E., Chwała, B., Moszyńska-Dumara, M., et al. (2015). Influence of candidate polymorphisms on the dipeptidyl peptidase IV and μ-opioid receptor genes expression in aspect of the β-casomorphin-7 modulation functions in autism. Peptides,65, 6–11.
Cornish, E. (2002). Gluten and casein free diets in autism: A study of the effects on food choice and nutrition. Journal of Human Nutrition & Dietetics,15, 261–269.
Dalton, N. R., Chandler, S., Turner, C., Charman, T., Pickles, A., Simonoff, E., et al. (2017). Measurement of urine indolylacroylglycine is not useful in the diagnosis or dietary management of autism. Autism Research,10, 408–413.
Dawson, G., Jones, E. J., Merkle, K., Venema, K., Lowy, R., Faja, S., et al. (2012). Early behavioral intervention is associated with normalized brain activity in young children with autism. Journal of the American Academy of Child and Adolescent Psychiatry,51, 1150–1159.
Dettmer, K., Hanna, D., Whetstone, P., Hansen, R., & Hammock, B. D. (2007). Autism and urinary exogenous neuropeptides: Development of an on-line SPE-HPLC-tandem mass spectrometry method to test the opioid excess theory. Analytical and Bioanalytical Chemistry,388, 1643–1651.
Díaz-Atienza, F., Serrano, S., González-Domenech, P. J., & García, C. (2012). Prevalence of feeding disorders, gastrointestinal disorders and recurrent infections in children with Autism Spectrum Disorders (ASD) compared with their healthy siblings. Revista de Psiquiatría Infantil,29, 11–16.
Dosman, C., Adams, D., Wudel, B., Vogels, L., Turner, J., & Vohra, S. (2013). Complementary, holistic, and integrative medicine: Autism spectrum disorder and gluten-and casein-free diet. Pediatric Review,34, e36–e41.
Elder, J. H., Kreider, C. M., Schaefer, N. M., & de Laosa, M. B. (2015). A review of gluten- and casein-free diets for treatment of autism: 2005–2015. Nutritional Dietary Supplements,7, 87–101.
Elder, J. H., Shankar, M., Shuster, J., Theriaque, D., Burns, S., & Sherrill, L. (2006). The gluten-free, casein-free diet in autism: Results of a preliminary double blind clinical trial. Journal of Autism and Developmental Disorders,36, 413–420.
El-Rashidy, O., El-Baz, F., El-Gendy, Y., Khalaf, R., Reda, D., & Saad, K. (2017). Ketogenic diet versus gluten free casein free diet in autistic children: A case–control study. Metabolic Brain Disease,32, 1935–1941.
Geier, D. A., Kern, J. K., & Geier, M. R. (2014). A Comparison of the Autism Treatment Evaluation Checklist (ATEC) and the Childhood Autism Rating Scale (CARS) for the quantitative evaluation of autism. Journal of Mental Health Research in Intellectual Disabilities,6, 255–267.
Ghalichi, F., Ghaemmaghami, J., Malek, A., & Ostadrahimi, A. (2016). Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: A randomized clinical trial. World Journal of Pediatrics,12, 436–442.
Gillberg, C., & Schaumann, H. (1981). Infantile autism and puberty. Journal of Autism and Developmental Disorders,11, 365–371.
González-Domenech, P. J., Díaz, F., García, C., Serrano, S., Herreros, O., Gutierrez-Rojas, L., et al. (2019). Influence of a gluten-free, casein-free diet on behavioral disturbances in children and adolescents diagnosed with autism spectrum disorder: A 3-month follow-up pilot study. Journal of Mental Health Research in Intellectual Disabilities. https://doi.org/10.1080/19315864.2019.1654574.
Grimaldi, R., Gibson, G. R., Vulevic, J., Giallourou, N., Castro-Mejía, J. L., Hansen, L. H., et al. (2018). A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome,6, 133.
Hediguer, M. L., England, L. J., Molloy, C. A., Yu, K. F., Manning-Courtney, P., & Mills, J. L. (2008). Reduced bone cortical thickness in boys with autism or autism spectrum disorder. Journal of Autism and Developmental Disorders,38, 848–856.
Hirsch, L. E., & Pringsheim, T. (2016). Aripiprazole for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews,26, CD009043.
Hunter, L. C., O’Hare, A., Herron, W. J., Fisher, L. A., & Jones, G. E. (2003). Opioid peptides and dipeptidyl peptidase in autism. Developmental Medicine and Child Neurology,45, 121–128.
Hyman, S. L., Stewart, P. A., Foley, J., Cain, U., Peck, R., Morris, D. D., et al. (2016). The gluten-free/casein-free diet: A double-blind challenge trial in children with autism. Journal of Autism and Developmental Disorders,46, 205–220.
ICD-10. (1992). Classifications of mental and behavioural disorder: Clinical descriptions and diagnostic guidelines. Geneva: World Health Organization.
Jarmolowska, B., Bukalo, M., Fiedorowicz, E., Cieślińska, A., Kordulewska, N. K., Moszyńska, M., et al. (2019). Role of milk-derived opioid peptides and proline dipeptidyl peptidase-4 in Autism Spectrum Disorders. Nutrients,11, E87.
Johnson, C. R., Handen, B. L., Zimmer, M., Sacco, K., & Turner, K. (2011). Effects of gluten free/casein free diet in young children with autism: A pilot study. Journal of Developmental and Physical Disabilities,23, 213–225.
Kanner, L. (1971). Follow-up study of eleven autistic children originally reported in 1943. Journal of Autism & Childhood Schizophrenia,1, 119–145.
Knivsberg, A. M., Reichelt, K. L., Høien, T., & Nødland, M. (2002). A randomised, controlled study of dietary intervention in autistic syndromes. Nutritional Neuroscience,5, 251–261.
Knivsberg, A. M., Reichet, K. L., Nodland, M., & Hoien, T. (1995). Autistic syndromes and diet: A follow-up study. Scandinavian Journal of Educational Research,39, 223–236.
Knivsberg, A. M., Wiig, K., Lind, G., Nødland, M., & Reichelt, K. L. (1990). Dietary intervention in autistic syndromes. Brain Dysfunction,3, 315–317.
Konstantynowicz, J., Nguyen, T. V., Kaczmarski, M., Jamiolkowsky, J., Piotrowska-Jastrzebska, J., & Seeman, E. (2007). Fractures during growth: Potential role of a milk-free diet. Osteoporosis International,18, 1601–1607.
Kumar, P., O’Donoghue, P., Stenson, K., & Dawson, A. (1979). Reintroduction of gluten in adults and children with treated celiac disease. Gut,20, 743–749.
Lange, K. W., Hauser, J., & Reissmann, A. (2015). Gluten-free and casein-free diets in the therapy of autism. Current Opinion in Clinical Nutrition & Metabolic Care,18, 572–575.
Le Menn-Tripi, C., Vachaud, A., Defas, N., Malvy, J., Roux, S., & Bonnet-Brilhault, F. (2019). Sensory-psychomotor evaluation in autism: A new tool for functional diagnosis. Encephale,45, 312–319.
Lucarelli, S., Frediani, T., Zingoni, A. M., Ferruzzi, F., Giardini, O., Quintieri, F., et al. (1995). Food allergy and infantile autism. Panminerva Medica,37, 137–141.
Maestro, S., Casella, C., Milone, A., Muratori, F., & Palacio-Espasa, F. (1999). Study of the onset of autism through home movies. Psychopathology,32, 292–300.
Mahapatra, S., Vyshedsky, D., Martinez, S., Kannel, B., Braverman, J., Edelson, S. M., et al. (2018). Autism treatment Evaluation Checklist (ATEC) norms: A “growth chart” for ATEC score changes as a function of age. Children (Basel),5, 25.
Marí-Bauset, S., Zazpe, I., Mari-Sanchis, A., Llopis-González, A., & Morales-Suárez-Varela, M. (2014). Evidence of the gluten-free and casein-free diet in autism spectrum disorders: A systematic review. Journal of Child Neurology,29, 1–10.
Millward, C., Ferriter, M., Calver, S., & Connell-Jones, G. (2008). Gluten and casein free diets for autistic spectrum disorder. Cochrane Database of Systematic Review,16, CD003498.
Monti, G., Libanore, V., Marinaro, L., Lala, R., Miniero, R., & Savino, F. (2007). Multiple bone fracture in an 8 years old child with cow’s milk allergy and inappropriate calcium supplementation. Annals of Nutrition & Metabolism,51, 228–231.
Mulloy, A., Lang, R., O´Reilly, M., Sigafoos, J., Lancioni, G. L., & Rispoli, M. (2010). Gluten-free and casein-free diet in the treatment of autism spectrum disorders: A systematic review. Research in Autism Spectrum Disorders,4, 328–339.
Navarro, F., Pearson, D. A., Fatheree, N., Mansour, R., Hashmi, S. S., & Rhoads, J. M. (2015). Are ‘leaky gut’ and behavior associated with gluten and dairy containing diet in children with autism spectrum disorders? Nutritional Neuroscience,18, 177–185.
Neumeyer, A. M., Gates, A., Ferrone, C., Lee, H., & Misra, M. (2013). Bone density in peripubertal boys with autism spectrum disorders. Journal of Autism and Developmental Disorders,43, 1623–1629.
Nygaard, H. A. (2010). Pain in people with dementia and impaired verbal communication. Journal of Pain and Palliative Care Pharmacotherapy,24, 414–426.
Oneal, B. J., Reeb, R. N., Korte, J. R., & Butter, E. J. (2006). Assessment of home-based behavior modification programs for autistic children: Reliability and validity of the behavioral summarized evaluation. Journal of Prevention & Intervention in the Community,32, 25–39.
Owen-Smith, A. A., Bent, S., Lynch, F. L., Coleman, K. J., Yau, V. M., Pearson, K. A., et al. (2015). Prevalence and predictors of complementary and alternative medicine use in a large insured sample of children with autism spectrum disorders. Research in Autism Spectrum Disorders,17, 40–51.
Pedersen, L., Parlar, S., Kvist, K., Whiteley, P., & Shattock, P. (2013). Data mining the ScanBrit study of a gluten and casein-free dietary intervention for children with autism spectrum disorders: Behavioural and psychometric measures of dietary response. Nutritional Neuroscience,17, 207–213.
Pennesi, C. M., & Klein, L. C. (2012). Effectiveness of the gluten-free, casein-free diet for children diagnosed with autism spectrum disorder: Based on parental report. Nutritional Neuroscience,15, 85–91.
Perrin, J. M., Coury, D. L., Hyman, S. L., Cole, L., Reynolds, A. M., & Clemons, T. (2012). Complementary and alternative medicine use in a large pediatric autism sample. Pediatrics,130(Suppl. 2), S77–S82.
Piwowarczyk, A., Horvath, A., Łukasik, J., Pisula, E., & Szajewska, H. (2018). Gluten- and casein-free diet and autism spectrum disorders in children: A systematic review. European Journal of Nutrition,57, 433–440.
Pusponegoro, H. D., Ismael, S., Firmansyah, A., Sastroasmoro, S., & Vandenplas, Y. (2015). Gluten and casein supplementation does not increase symptoms in children with autism spectrum disorder. Acta Paediatrica,104, e500–e505.
Reichelt, K. L., & Knivsberg, A. M. (2003). Can the pathophysiology of autism be explained by the nature of the discovered urine peptides? Nutritional Neuroscience,6, 19–28.
Reichelt, K. L., Knivsberg, A. M., Lind, G., & Nodland, M. (1991). Probable etiology and treatment of childhood autism. Brain Dysfunction,4, 308–319.
Reichelt, K. L., Saelid, G., Lindback, T., & Boler, J. B. (1986). Childhood autism: A complex disorder. Biological Psychiatry,21, 1279–1290.
Reichelt, K. L., Tveiten, D., Knivsberg, A. M., & Bronstad, G. (2012). Peptide’s role in autism with emphasis on exorphins. Microbial Ecology in Health and Disease,23, 18958.
Reichow, B., Barton, E. E., Boyd, B. A., & Hume, K. (2014). Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD): A systematic review. Campbell Systematic Reviews. https://doi.org/10.4073/csr.2014.9.
Rimland, B., & Edelson, M. (1999). Autism treatment evaluation checklist. San Diego, CA: Autism Research Institute.
Rubenstein, E., Schieve, L., Bradley, C., DiGuiseppi, C., Moody, E., Thomas, K., et al. (2018). The prevalence of gluten free diet use among preschool children with autism spectrum disorder. Autism Research,11, 185–193.
Sathe, N., Andrews, J. C., McPheeters, M. L., & Warren, Z. E. (2017). Nutritional and dietary interventions for autism spectrum disorder: A systematic review. Pediatrics,139, e20170346.
Schopler, E., Reichler, R. J., DeVellis, R. F., & Daly, K. (1980). Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). Journal of Autism and Developmental Disorders,10, 91–103.
Seung, H. K., Rogalski, Y., Shankar, M., & Elder, J. (2007). The gluten- and casein-free diet and autism: Communication outcomes from a preliminary double-blind clinical trial. Journal of Medical Speech-Language Pathology,15, 337–339.
Sokolov, O., Kost, N., Andreeva, O., Korneeva, E., Meshavkin, V., Tarakanova, Y., et al. (2014). Autistic children display elevated urine levels of bovine casomorphin-7 immunoreactivity. Peptides,56, 68–71.
Trudeau, M. S., Madden, R. F., Parnell, J. A., Gibbard, W. B., & Shearer, J. (2019). Dietary and supplement-based complementary and alternative medicine use in pediatric autism spectrum disorder. Nutrients,11, 1783.
Tveiten, D., Finvold, A., Andersson, M., & Reichelt, K. L. (2014). Peptides and exorphins in the autism spectrum. Open Journal of Psychiatry,4, 275–287.
Wang, L., Angley, M. T., Gerber, J. P., Young, R. L., Abarno, D. V., McKinnon, R. A., et al. (2009). Is urinary indolyl-3-acryloylglycine a biomarker for autism with gastrointestinal symptoms? Biomarkers,14, 596–603.
Whiteley, P. (2015). Nutritional management of (some) autism: A case for gluten- and casein-free diets? Proceedings of the Nutrition Society,74, 202–207.
Whiteley, P., Haracopos, D., Knivsberg, A. M., Reichelt, K. L., Parlar, S., Jacobsen, J., et al. (2010a). The ScanBrit randomised, controlled, single-blind study of a gluten and casein-free dietary intervention for children with autism spectrum disorders. Nutritional Neuroscience,13, 87–100.
Whiteley, P., Rodgers, J., Savery, D., & Shattock, P. (1999). A gluten-free diet as an intervention for autism and associated spectrum disorders: Preliminary findings. Autism,3, 45–65.
Whiteley, P., Shattock, P., Carr, K., Hooper, M., & Todd, L. (2010b). How could a gluten and casein-free diet ameliorate symptoms associated with autism spectrum conditions? Autism Insights,2, 39–53.
Whiteley, P., Shattock, P., Knivsberg, A. M., Seim, A., Reichelt, K. L., Todd, L., et al. (2013). Gluten- and casein-free dietary intervention for autism spectrum conditions. Frontiers in Human Neuroscience,6, 344.
Acknowledgments
We thank University of Granada, in particular, Professor Manuel Gurpegui Fernández de Legaria for his advices and suggestions regarding this manuscript. The authors acknowledge Nutraceutical Translations for translating this manuscript into English.
Author information
Authors and Affiliations
Contributions
PJG-D has participated in the design of the study, collected the data, participated in the statistical analysis, the interpretation of the data and the drafting of the article; he approved the final version of the manuscript. FDA, CGP, JMM-O, LG-R has participated in the statistical analysis, the interpretation of the data and the drafting of the article; she approved the final version of the manuscript. MLFS has participated in the design of the study, the statistical analysis, the interpretation of the data and the drafting of the article; he approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants
The investigation was carried out in accordance with the Helsinki Declaration of 1975, as revised in 2013. The research protocol was approved by the local Institutional Review Board.
Informed Consent
All patients gave written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
González-Domenech, P.J., Díaz Atienza, F., García Pablos, C. et al. Influence of a Combined Gluten-Free and Casein-Free Diet on Behavior Disorders in Children and Adolescents Diagnosed with Autism Spectrum Disorder: A 12-Month Follow-Up Clinical Trial. J Autism Dev Disord 50, 935–948 (2020). https://doi.org/10.1007/s10803-019-04333-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-019-04333-1