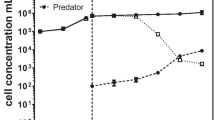
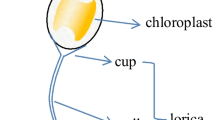
Abstract
Consumption of microalgae, as prey, by predatory zooplankton is a major ecological process in aquatic environments. The presence of predators in large-scale cultivation, such as in open ponds, results in a devastating loss of microalgal biomass, often referred to as a “pond crash.” Reported biomass losses of 20–30% due to predator invasion in open cultivation systems is one of the bottlenecks in achieving a desired economically viable system. Many commercial scale algal cultivation setups have reported clearance of prey within 2–5 days after detection of predators. Knowledge of how to monitor and manage algal pests is limited. Research to date is largely driven towards the development of predator mitigation strategies, whereas monitoring is mainly limited to traditional (direct) methods such as microscopy- and oligonucleotide-based screening. Use of online and real-time measures for in situ estimation of microalgal grazing is sparsely reported. We suggest that more knowledge about microalgal grazing at the pond level is required for the development of indirect screening measures, based on unique features of microalgal prey and predator interactions, to enable online monitoring. This article systematically reviews the current status of available methods, both at laboratory and field level, for early detection of microalgal grazing.
Similar content being viewed by others
References
Bartley ML, Boeing WJ, Corcoran AA, Holguin FO, Schaub T (2013) Effects of salinity on growth and lipid accumulation of biofuel microalga Nannochloropsis salina and invading organisms. Biomass Bioenergy 54:83–88
Bilanovic D, Andargatchew A, Kroeger T, Shelef G (2009) Freshwater and marine microalgae sequestering of CO2 at different C and N concentrations – response surface methodology analysis. Energy Convers Manag 50:262–267
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Borowitzka MA (2013) Energy from microalgae: a short history. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 1–15
Borowitzka MA, Moheimani NR (2013) Open pond culture systems. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 133–152
Bravo I, Figueroa R (2014) Towards an ecological understanding of dinoflagellate cyst functions. Microorganisms 2:11–32
Brussaard CP (2004) Viral control of phytoplankton populations—a review. J Eukaryot Microbiol 51:125–138
Carney LT, Lane TW (2014) Parasites in algae mass culture. Front Microbiol 5:278–286
Carney LT, Reinsch SS, Lane PD, Solberg OD, Jansen LS, Williams KP, Trent JD, Lane TW (2014) Microbiome analysis of a microalgal mass culture growing in municipal wastewater in a prototype OMEGA photobioreactor. Algal Res 4:52–61
Carney LT, Wilkenfeld JS, Lane PD, Solberg OD, Fuqua ZB, Cornelius NG, Gillespie S, Williams KP, Samocha TM, Lane TW (2016) Pond crash forensics: presumptive identification of pond crash agents by next generation sequencing in replicate raceway mass cultures of Nannochloropsis salina. Algal Res 17:341–347
Collins AM, Jones HD, McBride RC, Behnke C, Timlin JA (2014) Host cell pigmentation in Scenedesmus dimorphus as a beacon for nascent parasite infection. Biotechnol Bioeng 111:1748–1757
Crofts AR (1967) Amine uncoupling of energy transfer in chloroplasts. I. Relation to ammonium ion uptake. J Biol Chem 242:3352–3359
Dagenais-Bellefeuille S, Morse D (2013) Putting the N in dinoflagellates. Front Microbiol 4:369–683
Damiani MC, Leonardi PI, Pieroni OI, Cáceres EJ (2006) Ultrastructure of the cyst wall of Haematococcus pluvialis (Chlorophyceae): wall development and behaviour during cyst germination. Phycologia 45:616–623
Davis R, Markham J, Kinchin C, Grundl N, Tan EC, Humbird D (2016) Process design and economics for the production of algal biomass: algal biomass production in open pond systems and processing through dewatering for downstream conversion. Laboratory NREL, Golden, Colorado. NREL/TP-5100-64772 pp 1–112
Dawidziuk A, Popiel D, Luboinska M, Grzebyk M, Wisniewski M, Koczyk G (2017) Assessing contamination of microalgal astaxanthin producer Haematococcus cultures with high-resolution melting curve analysis. Microb Genet 58:277–285
Day JG, Thomas NJ, Achilles-Day UE, Leakey RJ (2012) Early detection of protozoan grazers in algal biofuel cultures. Bioresour Technol 114:715–719
Day JG, Gong Y, Hu Q (2017) Microzooplanktonic grazers – a potentially devastating threat to the commercial success of microalgal mass culture. Algal Res 27:356–365
Dean AP, Nicholson JM, Sigee DC (2012) Changing patterns of carbon allocation in lake phytoplankton: an FTIR analysis. Hydrobiologia 684:109–127
Deglint JL, Jin C, Wong A (2018) Investigating the automatic classification of algae using fusion of spectral and morphological characteristics of algae via deep residual learning. https://arxiv.org/abs/1810.10889
Deore P, Beardall J, Noronha S (2020a) Non-photochemical quenching, a non-invasive probe for monitoring microalgal grazing: influence of grazing-mediated total ammonia-nitrogen. Appl Phycol 1:32–43
Deore P, Karthikaichamy A, Beardall J, Noronha S (2020b) Non-photochemical quenching, a non-invasive probe for monitoring microalgal grazing: an early indicator of predation by Oxyrrhis marina and Euplotes sp. Appl Phycol 1:20–31
Di Caprio F (2020) Methods to quantify biological contaminants in microalgae cultures. Algal Res 49:101943
Ding Y, Peng X, Wang Z, Wen X, Geng Y, Zhang D, Li Y (2018) Occurrence and characterization of an epibiotic parasite in cultures of oleaginous microalga Graesiella sp. WBG-1. J Appl Phycol 30:819–830
Ferrell J, Sarisky-Reed V (2010) National algal biofuels technology roadmap. U.S. Department of Energy Biomass program, Maryland, USA. DOE/EE-03324329 pp 1–124.
Flynn KJ, Kenny P, Mitra A (2017) Minimising losses to predation during microalgae cultivation. J Appl Phycol 29:1829–1840
Fott B (1967) Phlyctidium scenedesmi spec. nova, a new chytrid destroying mass cultures of algae. Z Allgem Mikrobiol 7:97–102
Fulbright SP, Dean MK, Wardle G, Lammers PJ, Chisholm S (2014) Molecular diagnostics for monitoring contaminants in algal cultivation. Algal Res 4:41–51
Fundel B, Stich H, Schmid H, Maier G (1998) Can phaeopigments be used as markers for Daphnia grazing in Lake Constance. J Plankton Res 20:1449–1462
Gachon CM, Sime-Ngando T, Strittmatter M, Chambouvet A, Kim GH (2010) Algal diseases: spotlight on a black box. Trends Plant Sci 15:633–640
Ganuza E, Sellers CE, Bennett BW, Lyons EM, Carney LT (2016) A novel treatment protects Chlorella at commercial scale from the predatory bacterium Vampirovibrio chlorellavorus. Front Microbiol 7:848–861
Gerphagnon M, Latour D, Colombet J, Sime-Ngando T (2013) A double staining method using SYTOX green and calcofluor white for studying fungal parasites of phytoplankton. Appl Environ Microbiol 79:3943–3951
Giordano M, Ratti S, Domenighini A, Vogt F (2009) Spectroscopic classification of 14 different microalga species: first steps towards spectroscopic measurement of phytoplankton biodiversity. Plant Ecol Divers 2:155–164
Guo F, Kainz M, Sheldon F, Bunn S (2016) The importance of high-quality algal food sources in stream food webs - current status and future perspectives. Freshw Biol 61:815–831
Gutman J, Zarka A, Boussiba S (2009) The host-range of Paraphysoderma sedebokerensis, a chytrid that infects Haematococcus pluvialis. Eur J Phycol 44:509–514
Hansen PJ, BjØrnsen PK, Hansen BW (2000) Zooplankton grazing and growth: scaling within the 2–2,000-μm body size range. Limnol Oceanogr 45:1891–1891
Havlik I, Reardon KF, Ünal M, Lindner P, Prediger A, Babitzky A, Beutel S, Scheper T (2013) Monitoring of microalgal cultivations with on-line, flow-through microscopy. Algal Res 2:253–257
Jeffrey LCW (2011) The occurrence of ergosterol and (22E,24R)-24-ethylcholesta-5,7,22-trien-3β-ol in the unicellular chlorophyte Dunaliella tertiolecta. Can J Chem 57:2569–2571
Kansiz M, Heraud P, Wood B, Burden F, Beardall J, McNaughton D (1999) Fourier transform infrared microspectroscopy and chemometrics as a tool for the discrimination of cyanobacterial strains. Phytochemistry 52:407–417
Karuppasamy S, Musale AS, Soni B, Bhadra B, Gujarathi N, Sundaram M, Sapre A, Dasgupta S, Kumar C (2018) Integrated grazer management mediated by chemicals for sustainable cultivation of algae in open ponds. Algal Res 35:439–448
Knoshaug EP, Wolfrum E, Laurens LM, Harmon VL, Dempster TA, McGowen J (2018) Unified field studies of the algae testbed public-private partnership as the benchmark for algae agronomics. Sci Data 5:180267–180277
Lammers PJ, Huesemann M, Boeing W, Anderson DB, Arnold RG, Bai X, Bhole M, Brhanavan Y, Brown L, Brown J (2017) Review of the cultivation program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Res 22:166–186
Lane T, Poorey K, Geng H, Curtis DJ, Carney LT (2016) Algal crop protection strategies and technologies. Sandia National Laboratory, Livermore, United States. SAND2016-8236C
Ma M, Yuan D, He Y, Park M, Gong Y, Hu Q (2017) Effective control of Poterioochromonas malhamensis in pilot-scale culture of Chlorella sorokiniana GT-1 by maintaining CO2-mediated low culture pH. Algal Res 26:436–444
Ma M, Gong Y, Hu Q (2018) Identification and feeding characteristics of the mixotrophic flagellate Poterioochromonas malhamensis, a microalgal predator isolated from outdoor massive Chlorella culture. Algal Res 29:142–153
Maes D, Reichardt TA, Jensen TJ, Dempster TA, McGowen JA, Poorey K, Hipple T, Lane T, Timlin JA (2018) Spectroradiometric detection of competitors and predators in algal ponds. Sandia National Laboratory, Albuquerque,United States. SAND2018-6164C
Mansour MP, Volkman JK, Jackson AE, Blackburn SI (2002) The fatty acid and sterol composition of five marine dinoflagellates. J Phycol 35:710–720
McBride RC, Lopez S, Meenach S, Burnett M, Lee PA, Nohilly F, Behnke C (2014) Contamination management in low cost open algae ponds for biofuels production. Ind Biotechnol 10:221–227
McGowen J, Knoshaug EP, Laurens LM, Dempster TA, Pienkos PT, Wolfrum E, Harmon VL (2017) The Algae Testbed Public-Private Partnership (ATP3) framework; establishment of a national network of testbed sites to support sustainable algae production. Algal Res 25:168–177
Montagna PA (1995) Rates of metazoan meiofaunal microbivory: a review. Vie et Milieu 45:1–10
Moreno-Garrido I, Cañavate JP (2001) Assessing chemical compounds for controlling predator ciliates in outdoor mass cultures of the green algae Dunaliella salina. Aquac Eng 24:107–114
Müller P, Li X, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Murphy TE, Macon K, Berberoglu H (2013) Rapid algal culture diagnostics for open ponds using multispectral image analysis. Biotechnol Prog 30:233–240
Natchimuthu S, Chinnaraj P, Parthasarathy S, Senthil K (2013) Automatic identification of algal community from microscopic images. Bioinf Biol Insights 7:327–334
Park S-H, Steichen SA, Li X, Ogden K, Brown JK (2019) Association of Vampirovibrio chlorellavorus with decline and death of Chlorella sorokiniana in outdoor reactors. J Appl Phycol 31:1131–1142
Post FJ, Borowitzka LJ, Borowitzka MA, Mackay B, Moulton T (1983) The protozoa of a Western Australian hypersaline lagoon. Hydrobiologia 105:95–113
Poulton NJ, Martin JL (2010) Imaging flow cytometry for quantitative phytoplankton analysis-FlowCAM. In: Karlson B, Cusack C, Bresnan E (eds) Microscopic and molecular methods for quantitative phytoplankton analysis. UNESCO, Paris, pp 47–53
Rasconi S, Jobard M, Jouve L, Sime-Ngando T (2009) Use of calcofluor white for detection, identification, and quantification of phytoplanktonic fungal parasites. Appl Environ Microbiol 75:2545–2553
Ratti S, Knoll AH, Giordano M (2013) Grazers and phytoplankton growth in the oceans: an experimental and evolutionary perspective. PLoS One 8:e77349
Reese KL, Fisher CL, Lane PD, Jaryenneh JD, Moorman MW, Jones AD, Frank M, Lane TW (2019) Chemical profiling of volatile organic compounds in the headspace of algal cultures as early biomarkers of algal pond crashes. Sci Rep 9:1–10
Reichardt TA, Collins AM, McBride RC, Behnke CA, Timlin JA (2014) Spectroradiometric monitoring for open outdoor culturing of algae and cyanobacteria. Appl Opt 53:31–45
Reichardt TA, Maes D, Jensen T, Dempster TA, McGowen JA, Poorey K, Curtis DJ, Lane TW, Timlin J (2020) Spectroradiometric monitoring of competitor diatoms and the grazer Poteriochromonas in algal cultures. Algal Res 51:102020
Richardson JW, Johnson MD, Zhang X, Zemke P, Chen W, Hu Q (2014) A financial assessment of two alternative cultivation systems and their contributions to algae biofuel economic viability. Algal Res 4:96–104
Richmond A (ed) (2004) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Science Ltd, Oxford
Rogers JN, Rosenberg JN, Guzman BJ, Oh VH, Mimbela LE, Ghassemi A, Betenbaugh MJ, Oyler GA, Donohue MD (2014) A critical analysis of paddlewheel-driven raceway ponds for algal biofuel production at commercial scales. Algal Res 4:76–88
Ruiz J, Olivieri G, de Vree J, Bosma R, Willems P, Reith J, Eppink M, Kleinegris D, Wijffels R, Barbosa M (2016) Towards industrial products from microalgae. Energy Environ Sci 9:3036–3043
Samantaray A, Yang B, Dietz JE, Min BC (2018) Algae detection using computer vision and deep learning. https://arxiv.org/abs/1811.10847
Sandnes JM, Ringstad T, Wenner D, Heyerdahl PH, Kallqvist T, Gislerød HR (2006) Real-time monitoring and automatic density control of large-scale microalgal cultures using near infrared (NIR) optical density sensors. J Biotechnol 122:209–215
Schroeder DC, Oke J, Hall M, Malin G, Wilson WH (2003) Virus succession observed during an Emiliania huxleyi bloom. Appl Environ Microbiol 69:2484–2490
Smith VH, Foster BL, Grover JP, Holt RD, Leibold MA, deNoyelles F (2005) Phytoplankton species richness scales consistently from laboratory microcosms to the world's oceans. Proc Natl Acad Sci 102:4393–4396
Steedman HF (ed) (1976) Zooplankton fixation and preservation. UNESCO, Paris
Steichen S (2016) Tracking an algal predator: monitoring the dynamics of Vampirovibrio chlorellavorus in outdoor culture. MSc Thesis, The University of Arizona, USA 66 pp
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, United States
Strittmatter M, Guerra T, Silva J, Gachon CMM (2016) A new flagellated dispersion stage in Paraphysoderma sedebokerense, a pathogen of Haematococcus pluvialis. J Appl Phycol 28:1553–1558
Sudhakar K, Premalatha M (2015) Characterization of micro Aalgal biomass through FTIR/TGA /CHN Aanalysis: application to Scenedesmus sp. Energy Sources A 37:2330–2337
Urabe J, Kyle M, Makino W, Yoshida T, Andersen T, Elser JJ (2002) Reduced light increases herbivore production due to stoichiometric effects of light/nutrient balance. Ecology 83:619–627
Wang H, Zhang W, Chen L, Wang J, Liu T (2013) The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresour Technol 128:745–750
Wang Y, Castillo-Keller M, Eustance E, Sommerfeld M (2017) Early detection and quantification of zooplankton grazers in algal cultures by FlowCAM. Algal Res 21:98–102
Wang Y, Gong Y, Dai L, Sommerfeld M, Zhang C, Hu Q (2018) Identification of harmful protozoa in outdoor cultivation of Chlorella and the use of ultrasonication to control contamination. Algal Res 31:298–310
Funding
Research by the authors was funded by Reliance Industries Limited, Mumbai, India and IITB-Monash Research Academy, Mumbai, India. (Fund code–IMURA0303)
Author information
Authors and Affiliations
Contributions
Pranali Deore conceptualized and prepared manuscript. John Berdall corrected, improved, and critically reviewed the manuscript. Santosh Noronha critically reviewed and corrected the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deore, P., Beardall, J. & Noronha, S. A perspective on the current status of approaches for early detection of microalgal grazing. J Appl Phycol 32, 3723–3733 (2020). https://doi.org/10.1007/s10811-020-02241-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02241-x