Abstract
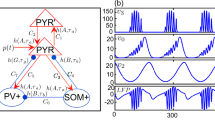
Epilepsy is one of the most common neurological disorders and is characterized by recurrent seizures. We use theoretical neuroscience tools to study brain dynamics during seizures. We derive and simulate a computational model of a network of hippocampal neuronal populations. Each population within the network is based on a model that has been shown to replicate the electrophysiological dynamics observed during seizures. The results provide insights into possible mechanisms for seizure spread. We observe that epileptiform activity remains localized to a pathological region when a global connectivity parameter is less than a critical value. After establishing the critical value for seizure spread, we explored how to correct the effect by altering particular synaptic gains. The spreading of seizures is quantified using numerical methods for seizure detection. The results from this study provide a new avenue of exploration for seizure control.










Similar content being viewed by others
References
Ahuja, AK, Dorn, JD, Caspi, A, McMahon, MJ, Dagnelie, G, da Cruz, L, Stanga, P, Humayun, MS, & Greenberg, RJ (2010). Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. British Journal of Ophthalmology, 95(4), 539–543.
Australian Bureau of Statistics. http://www.abs.gov.au.
Bartolomei, F., Wendling, F., Bellanger, J. J., Regis, J., & Chauvel, P. (2001). Neural networks involving the medial temporal structures in temporal lobe epilepsy. Clinical Neurophysiology, 112, 1746–1760.
Bertman, E H (2009). Temporal lobe epilepsy: Where do the seizures really begin? Epilepsy & Behavior, 14, 32–37.
Clark, G. (2003). Cochlear Implants: Fundamentals and applications: Springer Vergas New York Inc.
Da Silva, D. F., Hoeks, A., Smits, H., & Zetterberg, L. H. (1974). Model of brain rhythmic activity. Kybernetik, 15(1), 27–37.
Deco, G., Jirka, V. K., Robinson, P. A., Breakspear, M., & Friston, K. (2008). The dynamic brain: From spiking neurons to neural masses and cortical fields. PLoS Computational Biology, 4(8), 1–35.
Esteller, R., Echauz, J., Tcheng, T., & Litt, B. (2001). Line length: An efficient feature for seizure onset detection. Proceedings of the IEEE EMBS Conference, 1707–1710.
Fisher, R. S., van Emde Boas, W., Blume, B., Elger, C., Genton, P., Lee, P., & Engel, Jr. J (2005). Epileptic seizures and epilepsy: Definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia, 46, 470–474.
FitzHugh, R. (1955). Mathematical models of threshold phenomena in the nerve membrane. Bulletin of Mathematical Biophysics, 17(4), 257–278.
Freestone, D. R., Aramd, P., Dewarc, M., Scerrif, K., Grayden, D. B., & Kadirkamanathand, V. (2011). A data-driven framework for neural field modeling. NeuroImage, 56(3), 1043–1058.
Grimbert, F., & Faugeras, O. (2006). Bifurcation analysis of jansen’s neural mass model. Neural Computation, 18(12), 3052– 3068.
Guo, L., Rivero, D., Dorado, J., Rabunal, J. R., & Pazos, A. (2010). Automatic epileptic seizure detection in EEGs based on line length feature and artificial neural networks. Journal of Neuroscience Methods, 191 (1), 101–109.
Han, C. L., Hu, W., Stead, M., Zhang, T., Zhang, J. G., Worrell, G. A., & Meng, F. G. (2014). Electrical stimulation of hippocampus for the treatment of refractory temporal lobe epilepsy. Brain Research Bulletin, 109, 13–21.
Hirtz, D., Thurman, D., Gwinn-Hardy, K., Mohammad, M., Chaudhuri, A. R., & Zalutsky, R. (2007). How common are the “common” neurologic disorders? Neurology, 68(5), 326–337.
Holtzheimer, P. E., & Mayberg, H. S. (2011). Deep brain stimulation for psychiatric disorders. Annual Reviews in the Neurosciences, 34, 289–307.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of Physiology, 117(4), 500–544.
Jansen, B. H., & Rit, V. G. (1995). Electroencephalogram and visual evoked potential generation in a mathematical model of coupled cortical columns. Biological Cybernetics, 4, 357–366.
Nunez, P. L., & Srinivasan, R. (2006). Electric fields of the brain, 2nd Edn. Oxford: Oxford University Press.
Rowe, D. L., Robinson, P. A., Rennie, C. J., Harris, A. W., Felmingham, K. L., Lazzaro, I. L., & Gordon, E. (2004). Neurophysiologically-based mean-field modelling of tonic cortical activity in post-traumatic stress disorder (PTSD), schizophrenia, first episode schizophrenia and attention deficit hyperactivity disorder (ADHD). Journal of Integrative Neuroscience, 3(4), 453–487.
Schelter, B., Mader, M., Mader, W., Sommerlade, L., Platt, B., Lai, Y. C., Gregory, C., & Tiel, M. (2014). Overarching framework for data-based modelling. Frontiers of Physics, 105, 30004.
Schevron, C. A., Weiss, S. A., McKhann, Jr G., Goodman, R. R., Yuste, R., Emerson, R. G., Trevelyan, A.J, & Evidence of an inhibitory restraint of seizure activity in humans (2012). Nature Communications. doi:10.1038/ncomms2056.
Sobayo, T., Fine, A. S., Gunnar, E., Kazlauskas, C., Nicholls, D., & Mogul, D. J. (2013). Synchrony dynamics across brain structures in limbic epilepsy vary between initiation and termination phases of seizures. IEEE Transactions on Biomedical Engineering, 60(3), 821–829.
Stead, M., Bower, M., Brinkmann, B. H., Lee, K., Marsh, W. R., Meyer, F. B., Litt, B., van Gompel, J., & Worrell, G. A. (2010). Microseizures and the spatiotemporal scales of human partial epilepsy. Brain: A Journal of Neurology, 133, 2789–2797.
Touboul, J., Wendling, F., Chauvel, P., & Faugeras, O. (2011). Neural mass activity, bifurcations, and epilepsy. Neural Computation, 23(12), 3232–3286.
Wang, Y., Goodfellow, M., Taylor, P. N., & Baier, G. (2014). Dynamic Mechanisms of Neocortical Focal Seizure Onset. PLoS Computational Biology, 10(8), e1003787.
Wendling, F., Bellanger, J. J., Bartolomei, F., & Chauvel, P. (2000). Relevance of nonlinear lumped-parameter models in the analysis of depth-EEG epileptic signals. Biological Cybernetics, 4, 367–378.
Wendling, F., Bartolomei, F., Bellanger, J. J., & Chauvel, P. (2002). Epileptic fast activity can be explained by a model of impaired GABAergic dendritic inhibition. European Journal of Neuroscience, 9, 1499–1508.
Wilson, H. R., & Cowan, J. D. (1972). Excitatory and inhibitory interactions in localized populations of model neurons. Biophysical Journal, 12(1), 1–24.
Acknowledgments
This research was supported by the Australian Research Council through the Discovery Early Career Researcher Award (DECRA) DE120102210. Dr Freestone acknowledges the support of the Australian-American Fulbright Association. Dr Freestone would also like to thank Prof Liam Paninski at Columbia University for support and guidance. The authors would also like to thank and acknowledge important input from Prof Mark Cook, Dr Andre Peterson, Prof David Grayden, Prof Anthony Burkitt and all the members of the NeuroEngineering lab at The University of Melbourne.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Action Editor: Steven J. Schiff
Rights and permissions
About this article
Cite this article
Kameneva, T., Ying, T., Guo, B. et al. Neural mass models as a tool to investigate neural dynamics during seizures. J Comput Neurosci 42, 203–215 (2017). https://doi.org/10.1007/s10827-017-0636-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-017-0636-x