Abstract
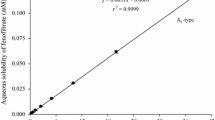
This study aimed to investigate the effect of hydroxypropyl methylcellulose on the complexation of fenofibrate and hydroxypropyl-β-cyclodextrin (HP-β-CD). Initially, phase solubility studies with an excess amount of drug in the HP-β-CD solutions with and without hydroxypropyl methylcellulose (HPMC) were investigated. Both of the binary and ternary complexes were prepared by ball-milling. The complexes were characterized by Fourier transform infrared spectroscopy (FI-IR), X-ray powder diffraction (XPRD), differential scanning calorimetry (DSC) and nuclear magnetic resonance spectroscopy (1H-NMR). The AL type phase-solubility diagram revealed that the complexes of fenofibrate and HP-β-CD were formed with molecular ratio of 1:1. The results of FT-IR, XPRD, DSC and 1H NMR analysis show the formulation of inclusion complexes. In conclusion, the interaction occurrs between fenofibrate and HP-β-CD in the complexes, and the existence of HPMC effectively improves the complexation efficiency and stability constant. The in vitro dissolution test suggests ternary complex is superior to binary complex in terms of the release of fenofibrate.






Similar content being viewed by others
References
Sanganwar, G.P., Gupta, R.B.: Dissolution-rate enhancement of fenofibrate by adsorption onto silica using supercritical carbon dioxide. Int. J. Pharm. 360, 213–218 (2008)
Steiner, G.: Fenofibrate for cardiovascular disease prevention in metabolic syndrome and type 2 diabetes mellitus. Am. J. Cardiol. 102, 28L (2008)
Huang, Q.P., Wang, J.X., Zhang, Z.B., Shen, Z.G., Chen, J.F., Yun, J.: Preparation of ultrafine fenofibrate powder by solidification process from emulsion. Int. J. Pharm. 368, 160–164 (2009)
Zhong, L., Zhu, X., Luo, X., Su, W.: Dissolution properties and physical characterization of telmisartan–chitosan solid dispersions prepared by mechanochemical activation. AAPS PharmSciTech. 14, 541–550 (2013)
Silva, C.V., Barbosa, J.A., Ferraz, M.S., Silva, N.H., Honda, N.K., Rabello, M.M., Hernandes, M.Z., Bezerra, B.P., Cavalcanti, I.M., Ayala, A.P.: Molecular modeling and cytotoxicity of diffractaic acid: HP-β-CD inclusion complex encapsulated in microspheres. Int. J. Biol. Macromol. 92, 494–503 (2016)
Aloisio, C., Antimisiaris, S.G., Longhi, M.R.: Liposomes containing cyclodextrins or meglumine to solubilize and improve the bioavailability of poorly soluble drugs. J. Mol. Liq. 229, 106–113 (2017)
Du, F., Meng, H., Xu, K., Xu, Y., Luo, P., Luo, Y., Lu, W., Huang, J., Liu, S., Yu, J.: Colloids: CPT loaded nanoparticles based on beta-cyclodextrin-grafted poly(ethylene glycol)/poly (L-glutamic acid) diblock copolymer and their inclusion complexes with CPT. Surf. B Biointerfaces 113, 230–236 (2014)
Pereira, R.A., Anconi, C.P., Nascimento Jr, C.S., De Almeida, W.B., Dos Santos, H.F.: Stability and spatial arrangement of the 2,4-dichlorophenoxyacetic acid and β-cyclodextrin inclusion compound: a theoretical study. Chem. Phys. Lett. 633, 158–162 (2015)
Jun, S.W., Kim, M.S., Kim, J.S., Park, H.J., Lee, S., Woo, J.S., Hwang, S.J.: Preparation and characterization of simvastatin/hydroxypropyl-beta-cyclodextrin inclusion complex using supercritical antisolvent (SAS) process. Eur. J. Pharm. Biopharm. 66, 413 (2007)
Fraceto, L.F., Grillo, R., Sobarzo-Sánchez, E.: Cyclodextrin inclusion complexes loaded in particles as drug carrier systems. Curr. Top. Med. Chem. 14, 518 (2014)
Liao, Y., Zhang, X., Li, C., Huang, Y., Lei, M., Yan, M., Zhou, Y., Zhao, C.: Inclusion complexes of HP-β-cyclodextrin with agomelatine: preparation, characterization, mechanism study and in vivo evaluation. Carbohydr. Polym. 147, 415–425 (2016)
Raza, A., Sun, H., Bano, S., Zhao, Y., Xu, X., Tang, J.: Preparation, characterization, and invitro anti-inflammatory evaluation of novel water soluble kamebakaurin/hydroxypropyl-β-cyclodextrin inclusion complex. J. Mol. Struct. 1130, 319–326 (2017)
Ribeiro, L., Loftsson, T., Ferreira, D., Veiga, F.: Investigation and physicochemical characterization of vinpocetine-sulfobutyl ether beta-cyclodextrin binary and ternary complexes. Chem. Pharm. Bull. 51, 914–922 (2003) (Tokyo)
Sauceau, M., Rodier, E., Fages, J.: Preparation of inclusion complex of piroxicam with cyclodextrin by using supercritical carbon dioxide. J. Supercrit. Fluids. 47, 326–332 (2008)
Rao, M., Bajaj, A., Khole, I., Munjapara, G., Trotta, F.: In vitro and in vivo evaluation of β-cyclodextrin-based nanosponges of telmisartan. J. Incl. Phenom. Macrocycl. Chem. 77(1-4), 135–145
Zhang, M., Li, H., Lang, B., O’Donnell, K., Zhang, H., Wang, Z., Dong, Y., Wu, C., Iii, R.O.W.: Formulation and delivery of improved amorphous fenofibrate solid dispersions prepared by thin film freezing. Eur. J. Pharm. Biopharm. 82, 534 (2012)
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59, 645–666 (2007)
Danciu, C., Soica, C., Oltean, M., Avram, S., Borcan, F., Csanyi, E., Ambrus, R., Zupko, I., Muntean, D., Dehelean, C.A.: Genistein in 1:1 inclusion complexes with ramified cyclodextrins: theoretical, physicochemical and biological evaluation. Int. J. Mol. Sci. 15, 1962–1982 (2014)
Ol’Khovich, M.V., Sharapova, A.V., Lavrenov, S.N., Blokhina, S.V., Perlovich, G.L.: Inclusion complexes of hydroxypropyl-β-cyclodextrin with novel cytotoxic compounds: Solubility and thermodynamic properties. Fluid Phase Equilib. 384, 68–72 (2014)
Da, Z., Jianqiang, Z., Kunming, J., Ke, L., Yangwei, C., Shaoping, P., Yi, J., Jun, L.: Preparation, characterisation and antitumour activity of β-, γ- and HP-β-cyclodextrin inclusion complexes of oxaliplatin. Spectrochim. Acta Part A. 152, 501–508 (2016)
Patel, A.R., Vavia, P.R.: Preparation and evaluation of taste masked famotidine formulation using drug/β-cyclodextrin/polymer ternary complexation approach. AAPS PharmSciTech. 9, 544–550 (2008)
Liu, B., Zhao, J., Liu, Y., Zhu, X., Zeng, J.: Physicochemical [corrected] properties of the inclusion complex of puerarin and glucosyl-β-cyclodextrin. J. Agric. Food Chem. 60, 12501 (2012)
Zhang, W., Gong, X., Cai, Y., Zhang, C., Yu, X., Fan, J., Diao, G.: Investigation of water-soluble inclusion complex of hypericin with β-cyclodextrin polymer. Carbohydr. Polym. 95, 366–370 (2013)
Chowdhry, B.Z., Leharne, S.A., Badwan, A.A.: Novel inclusion complex of ibuprofen tromethamine with cyclodextrins: physico-chemical characterization. J. Pharm. Biomed. Anal. 50, 449–458 (2009)
Roy, A., Saha, S., Roy, M.N.: Exploration of inclusion complexes of probenecid with α and β-cyclodextrins: Enhancing the utility of the drug. J. Mol. Struct. 1144, 103–111 (2017)
Sailaja, U., Thayyil, M.S., Kumar, N.S.K., Govindaraj, G.: Molecular dynamics of amorphous pharmaceutical fenofibrate studied by broadband dielectric spectroscopy. J. Pharm. Anal. 6, 165–170 (2016)
Linares, M.S., Longhi, M.R.: Effects of hydroxypropyl-beta-cyclodextrin on the chemical stability of a naphthoquinone in aqueous solutions. Pharmazie. 58, 32 (2003)
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21776254).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ding, X., Zheng, M., Lu, J. et al. Preparation and evaluation of binary and ternary inclusion complexes of fenofibrate/hydroxypropyl-β-cyclodextrin. J Incl Phenom Macrocycl Chem 91, 17–24 (2018). https://doi.org/10.1007/s10847-018-0793-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0793-1