Abstract
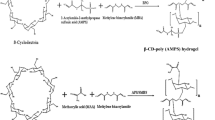
Nowadays, biomedical films containing drug carriers are preferred over conventional ones, since the protection of the injury and the therapy is joined within a single device. In the current work, we prepared polycaprolactone (PCL) composite films with β-cyclodextrin (βCD) or its epichlorohydrin crosslinked polymer (βCDP) as ibuprofen (Ibu) drug carrier. The composite films were prepared at different PCL/additive ratios (2, 5, 10 and 20 wt%). ATR-FTIR spectroscopy and water contact angle (WCA) measurements indicated a scarce presence of the additives on the surface. Cross-section scanning electron micrographs showed the presence of aggregates corresponding to βCD and βCDP in the inner regions of the films. The incorporation of βCD and βCDP into the PCL films did not affect their thermal properties as was determined from differential scanning calorimetry (DSC). PCL-films with 10 wt% of the inclusion complexes Ibu@βCD and Ibu@βCDP were prepared and the release studies were performed. At pH = 7.2, PCL-Ibu@βCDP composite film released 55% of Ibu within the first six hours; eight times the amount released by PCL-Ibu@βCD within the same time interval. A plausible mechanism for ibuprofen release is discussed based on the cross-section SEM micrographs of composite films.











Similar content being viewed by others
References
Ciccone, W.J., Motz, C., Bentley, C., Tasto, J.P.: Bioabsorbable implants in orthopaedics: New developments and clinical applications. J. Am. Acad. Orthop. Surg. 9, 280–288 (2001). https://doi.org/10.5435/00124635-200109000-00001
Sahoo, S., Sasmal, A., Nanda, R., Phani, A.R., Nayak, P.L.: Synthesis of chitosan–polycaprolactone blend for control delivery of ofloxacin drug. Carbohydr. Polym. 79, 106–113 (2010). https://doi.org/10.1016/j.carbpol.2009.07.042
Semnani, D., Nasari, M., Fakhrali, A.: PCL nanofibers loaded with beta-carotene: a novel treatment for eczema. Polym. Bull. 75, 2015–2026 (2018). https://doi.org/10.1007/s00289-017-2141-9
Massoumi, B., Taghavi, N., Ghamkhari, A.: Synthesis of a new biodegradable system based on β-cyclodextrin/iron oxide nanocomposite: application for delivery of docetaxel. Polym. Bull. (2020). https://doi.org/10.1007/s00289-020-03254-9
Ulery, B.D., Nair, L.S., Laurencin, C.T.: Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 49, 832–864 (2011). https://doi.org/10.1002/polb.22259
Middleton, J.C., Tipton, A.J.: Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 21, 2335–2346 (2000). https://doi.org/10.1016/S0142-9612(00)00101-0
Mondal, D., Griffith, M., Venkatraman, S.S.: Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 65, 255–265 (2016). https://doi.org/10.1080/00914037.2015.1103241
Malikmammadov, E., Tanir, T.E., Kiziltay, A., Hasirci, V., Hasirci, N.: PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 29, 863–893 (2018). https://doi.org/10.1080/09205063.2017.1394711
Mallakpour, S., Behranvand, V.: Polymeric nanoparticles: Recent development in synthesis and application. Express Polym. Lett. 10, 895–913 (2016). https://doi.org/10.3144/expresspolymlett.2016.84
Gidwani, B., Vyas, A.: Synthesis, characterization and application of Epichlorohydrin-β-cyclodextrin polymer. Colloids Surf., B. 114, 130–137 (2014). https://doi.org/10.1016/j.colsurfb.2013.09.035
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1753 (1998). https://doi.org/10.1021/CR970022C
Martin-Trasanco, R., Cao, R., Esparza-Ponce, H.E., Montero-Cabrera, M.E., Arratia-Pérez, R.: Reduction of Au(III) by a β-cyclodextrin polymer in acid medium. A stated unattainable reaction. Carbohydr. Polym. 175, 530–537 (2017). https://doi.org/10.1016/j.carbpol.2017.08.013
Martin-Trasanco, R., Anziani‐Ostuni, G., Esparza‐Ponce, H.E., Ortiz, P., Montero‐Cabrera, M.E., Oyarzún, D.P., Zúñiga, C., Pérez‐Donoso, J.M., Pizarro, G., del C., Arratia‐Pérez: R.: From concentrated dispersion to solid β‐cyclodextrin polymer‐capped silver nanoparticle formulation: A trojan horse against Escherichia coli. ChemistrySelect. 4, 10092–10096 (2019). https://doi.org/10.1002/slct.201901406
Martin, R., Sánchez, I., Cao, R., Rieumont, J.: Solubility and kinetic release studies of naproxen and ibuprofen in soluble epichlorohydrin-β-cyclodextrin polymer. Supramol. Chem. 18, 627–631 (2006). https://doi.org/10.1080/10610270601088073
Monteiro, A.P.F., Rocha, C.M.S.L., Oliveira, M.F., Gontijo, S.M.L., Agudelo, R.R., Sinisterra, R.D., Cortés, M.E.: Nanofibers containing tetracycline/β-cyclodextrin: Physico-chemical characterization and antimicrobial evaluation. Carbohydr. Polym. 156, 417–426 (2017). https://doi.org/10.1016/j.carbpol.2016.09.059
Canbolat, M.F., Celebioglu, A., Uyar, T.: Drug delivery system based on cyclodextrin-naproxen inclusion complex incorporated in electrospun polycaprolactone nanofibers. Colloids Surf., B. 115, 15–21 (2014). https://doi.org/10.1016/j.colsurfb.2013.11.021
Zhu, M., Wei, K., Lin, S., Chen, X., Wu, C.-C., Li, G., Bian, L.:Bioadhesive polymersome for localized and sustained drug delivery at pathological sites with harsh enzymatic and fluidic environment via supramolecular host-guest complexation. Small. 14, 1702288 (2018). https://doi.org/10.1002/smll.201702288
Varan, C., Bilensoy, E.: Development of implantable hydroxypropyl-β-cyclodextrin coated polycaprolactone nanoparticles for the controlled delivery of docetaxel to solid tumors. J. Incl. Phenom. Macrocycl. Chem. 80, 9–15 (2014). https://doi.org/10.1007/s10847-014-0422-6
DuBois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956). https://doi.org/10.1021/ac60111a017
Egyed, O.: Spectroscopic studies on β-cyclodextrin. Vib. Spectrosc. 1, 225–227 (1990). https://doi.org/10.1016/0924-2031(90)80041-2
Gautam, S., Dinda, A.K., Mishra, N.C.: Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C. (2013). https://doi.org/10.1016/j.msec.2012.12.015
Connors, K.A.: The stability of cyclodextrin complexes in solution. Chem. Rev. 97, 1325–1357 (1997). https://doi.org/10.1021/CR960371R
Coleman, A.W., Nicolis, I., Keller, N., Dalbiez, J.P.: Aggregation of cyclodextrins: An explanation of the abnormal solubility of?-cyclodextrin. J. Incl. Phenom. Mol. Recognit. Chem. 13, 139–143 (1992). https://doi.org/10.1007/BF01053637
Giordano, F., Novak, C., Moyano, J.R.: Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim. Acta. 380, 123–151 (2001). https://doi.org/10.1016/S0040-6031(01)00665-7
Hădărugă, N.G., Bandur, G.N., David, I., Hădărugă, D.I.: A review on thermal analyses of cyclodextrins and cyclodextrin complexes. Environ. Chem. Lett. 17, 349–373 (2019). https://doi.org/10.1007/s10311-018-0806-8
Heydari, A., Hassani, Y., Sheibani, H., Pardakhti, A.: Water-soluble β-cyclodextrin polymers as drug carriers to improve solubility, thermal stability and controlled release of nifedipine. Pharm. Chem. J. 51, 375–383 (2017). https://doi.org/10.1007/s11094-017-1617-0
Lizundia, E., Gómez-Galván, F., Pérez-Álvarez, L., León, L.M., Vilas, J.L.: Poly(L-lactide)/branched β-cyclodextrin blends: Thermal, morphological and mechanical properties. Carbohydr. Polym. 144, 25–32 (2016). https://doi.org/10.1016/j.carbpol.2016.02.043
Dong, T., Mori, T., Pan, P., Kai, W., Zhu, B., Inoue, Y.: Crystallization behavior and mechanical properties of poly(ε-caprolactone)/cyclodextrin biodegradable composites. J. Appl. Polym. Sci. 112, 2351–2357 (2009). https://doi.org/10.1002/app.29628
Ouellette, R.J., Rawn, J.D.: Carboxylic Acids and Esters. In: Ouellette, R.J. and Rawn, J.D.B.T.-P. of O.C. (eds.) Principles of Organic Chemistry. pp. 287–314. Elsevier, Boston: (2015)
Averous, L., Moro, L., Dole, P., Fringant, C.: Properties of thermoplastic blends: starch–polycaprolactone. Polymer. 41, 4157–4167 (2000). https://doi.org/10.1016/S0032-3861(99)00636-9
Magnusdottir, A., Másson, M., Loftsson, T.: Self Association and Cyclodextrin Solubilization of NSAIDs. J. Incl. Phenom. Macrocycl. Chem. 44, 213–218. https://doi.org/10.1023/A:1023079322024
Bettini, R., Colombo, P., Massimo, G., Catellani, P.L., Vitali, T.: Swelling and drug release in hydrogel matrices: polymer viscosity and matrix porosity effects. Eur. J. Pharm. Sci. 2, 213–219 (1994). https://doi.org/10.1016/0928-0987(94)90025-6
Albihn, P.: The 5-year accelerated ageing project for thermoset and thermoplastic elastomeric materials: A service life prediction tool. In: Coveney, V.A.B.T.-E. and C. (ed.) Elastomers and components, pp. 3–25. Elsevier (2006)
Acknowledgements
The authors acknowledge the support by internal projects of investigation number L19-01 and L217-15 from the Universidad Tecnológica Metropolitana and the program of Magíster en Química mención Tecnología de los Materiales held by the Universidad Tecnológica Metropolitana. Rudy Martin-Trasanco would like to acknowledge the support of the Fondo Nacional de Desarrollo Científico y Tecnológico Grant 11190555.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sepúlveda, F.A., Sánchez, J., Oyarzun, D.P. et al. Polycaprolactone and poly-β-cyclodextrin polymer blend: a Biopolymers composite film for drug release. J Incl Phenom Macrocycl Chem 102, 65–76 (2022). https://doi.org/10.1007/s10847-021-01101-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-021-01101-6