Abstract
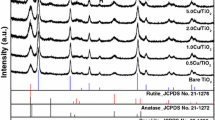
In this study, the short- and long-range chemical environments of Cu dopant in TiO2 photocatalyst have been investigated. The Cu-doped and undoped TiO2 specimens were prepared by the sol–gel approach employing CuSO4·5H2O and Ti(O-iPr)4 precursors and subjecting the dried gels to thermal treatment at 400 and 500 °C. The photocatalytic activity, investigated by methylene blue degradation under sunlight irradiation, showed a significantly higher efficiency of Cu-doped samples than that of pure TiO2. The X-ray diffraction results showed the presence of anatase phase for samples prepared at 400 and 500 °C. No crystalline CuSO4 phase was detected below 500 °C. It was also found that doping decreases the crystallite size in the (004) and (101) directions. Infrared spectroscopy results indicated that the chemical environment of sulfate changes as a function of thermal treatment, and UV–vis spectra showed that the band gap decreases with thermal treatment and Cu doping, showing the lowest value for the 400 °C sample. X-ray absorption fine structure measurements and analysis refinements revealed that even after thermal treatment and photocatalytic assays, the Cu2+ local order is similar to that of CuSO4, containing, however, oxygen vacancies. X-ray photoelectron spectroscopy data, limited to the near surface region of the catalyst, evidenced, besides CuSO4, the presence of Cu1+ and CuO phases, indicating the active role of Cu in the TiO2 lattice.








Similar content being viewed by others
References
Al-Kdasi A, Iris A, Saed K, Guan CT (2004) Glob Nest Int J 6:222
Di Paola A, Garcia-Lopez E, Ikeda S, Marci G, Ohtani B, Palmisano L (2002) Catal Today 75:87
Srinivas B, Shubhamangala B, Lalitha K, Reddy PAK, Kumari VD, Subrahmanyam M et al (2011) Photochem Photobiol 87:995
Tseng IH, Wu JCS, Chou HY (2004) J Catal 221:432
Gurman JS (1982) J Mater Sci 17:1541. doi:10.1007/BF00540779
Xiong L, Yang F, Yan L, Yan N, Yang X, Qiu M et al (2011) J Phys Chem Solids 72:1104
Carvalho HWP, Batista APL, Hammer P, Ramalho TC (2010) J Hazard Mater 184:273
Ravel B, Newville M (2005) J Synchrotron Radiat 12:537
Madarasz J, Braileanu A, Pokol G (2008) J Anal Appl Pyrolysis 82:292
Collins LW, Gibson EK, Wendlandt WW (1974) Thermochim Acta 9:15
Rao BR (1961) Acta Crystallogr 14:321
López-Ayala S, Rincón ME, Pfeiffer H (2009) J Mater Sci 44:4162. doi:10.1007/s10853-009-3617-2
Colon G, Maicu M, Hidalgo MC, Navio JA (2006) Appl Catal B 67:41
Moon J, Takagi H, Fujishiro Y, Awano M (2001) J Mater Sci 36:949. doi:10.1023/A:1004819706292
Nakamoto K (1986) Infrared and Raman spectra of inorganic and coordination compounds, 4th edn. Wiley, New York, p 232
Liao LF, Lien CF, Lin JL (2001) Phys Chem Chem Phys 3:3831
Joyner RW (1980) Chem Phys Lett 72:162
Moulder JF, Stickle WF, Sobol PE, Bomben KD (1992) Handbook of X-ray photoelectron spectroscopy Chastain J, editor. Physical Electronics Division, Eden Prairie, Perkin-Elmer Corporation, Minnesota
Bueno PR, Tararan R, Parra R, Joanni E, Ramirez MA, Ribeiro WC et al (2009) J Phys D 42:050404
Xin B, Wang P, Ding D, Liu J, Ren Z, Fu H (2008) Appl Surf Sci 254:2569
Acknowledgements
The authors are grateful to LNLS by the XAS beam time under the Project D04B-XAFS1-10796, and also to SOLEIL synchrotron for XRD and FTIR facilities.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Carvalho, H.W.P., Rocha, M.V.J., Hammer, P. et al. TiO2–Cu photocatalysts: a study on the long- and short-range chemical environment of the dopant. J Mater Sci 48, 3904–3912 (2013). https://doi.org/10.1007/s10853-013-7192-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7192-1