Abstract
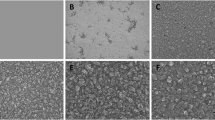
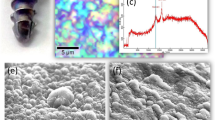
Diamond is an attractive material for biomedical implants. In this work, we investigate its capacity as a bone scaffold. It is well established that the bioactivity of a material can be evaluated by examining its capacity to form apatite-like calcium phosphate phases on its surface when exposed to simulated body fluid. Accordingly, polycrystalline diamond (PCD) and ultrananocrystalline diamond (UNCD) deposited by microwave plasma chemical vapour deposition were exposed to simulated body fluid and assessed for apatite growth when compared to the bulk silicon. Scanning electron microscopy and X-ray photoelectron spectroscopy showed that both UNCD and PCD are capable of acting as a bone scaffold. The composition of deposited apatite suggests that UNCD and PCD are suitable for in vivo implantation with UNCD possible favoured in applications where rapid osseointegration is essential.









Similar content being viewed by others
References
Baker K, Anderson M, Oehlke S, Astashkina A, Haikio D, Drelich J, et al. Growth, characterization and biocompatibility of bone-like calcium phosphate layers biomimetically deposited on metallic substratate. Mater Sci Eng, C. 2006;26(8):1351–60.
Stigter M, Bezemer J, de Groot K, Layrolle P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J Controlled Release. 2004;99(1):127–37. doi:10.1016/j.jconrel.2004.06.011.
Choi JM, Kim HE, Lee IS. Ion-beam-assisted deposition (IBAD) of hydroxyapatite coating layer on Ti-based metal substrate. Biomaterials. 2000;21(5):469–73.
Knabe C, Berger G, Gildenhaar R, Klar F, Zreiqat H. The modulation of osteogenesis in vitro by calcium titanium phosphate coatings Biomaterials. 2004;25(20):4911–9.
Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J Biomed Mater Res. 1990;24(6):721–34.
Coathup M, Blackburn J, Goodship A, Cunningham J, Smith T, Blunn G. Role of hydroxyapatite coating in resisting wear particle migration and osteolysis around acetabular components. Biomaterials. 2005;26(19):4161–9.
Sun LM, Berndt CC, Gross KA, Kucuk A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J Biomed Mater Res. 2001;58(5):570–92. doi:10.1002/jbm.1056.
Sun LM, Berndt CC, Khor KA, Cheang HN, Gross KA. Surface characteristics and dissolution behavior of plasma-sprayed hydroxyapatite coating. J Biomed Mater Res. 2002;62(2):228–36. doi:10.1002/jbm.10315.
Cheang P, Khor KA. Addressing processing problems associated with plasma spraying of hydroxyapatite coatings. Biomaterials. 1996;17(5):537–44. doi:10.1016/0142-9612(96)82729-3.
Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements as bone drug delivery systems: A review. J Controlled Release. 2006;113(2):102–10. doi:10.1016/j.jconrel.2006.04.007.
Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison with natural bone. Biomaterials. 2004;25(6):987–94.
Venkatesan P, Puvvada N, Dash R, Kumar BNP, Sarkar D, Azab B, et al. The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials. 2011;32(15):3794–806. doi:10.1016/j.biomaterials.2011.01.027.
Uskoković V, Uskoković D. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J Biomed Mater Res B Appl Biomater. 2011;96B:152–91.
Nath S, Basu B. Materials for orthopedic applications. In: Basu B, Katti D, Kumar A, editors. Advanced Biomaterials: Fundamentals, processing and applications. Hoboken: Wiley; 2009.
Klein C, Groot Kd. Implant systems based on bioactive ceramics. In: Heimke G, editor. Osseo-integrated implants: Implants in oral and ENT surgery. Boca Raton: CRC Press; 1990. p. 193–208.
Yoshinari M, Klinge B, Derand T. The biocompatibility (cell culture and histologic study) of hydroxyl-apatite-coated implants created by ion beam dynamic mixing. Clin Oral Implant Res. 1996;7:96–100.
Cook S, Thomas K, Brinker M. Bioactive ceramic coatings for orthopaedic and dental implant applications. Blood compatible materials and devices: Perspectives towards the 21st century. Lancaster: Technomic publishing company; 1991.
Coathup MJ, Blunn GW, Flynn N, Williams C, Thomas NP. A comparison of bone remodelling around hydroxyapatite-coated, porous-coated and grit-blasted hip replacements retrieved at post-mortem. Journal of Bone and Joint Surgery-British Volume. 2001;83B(1):118–23. doi:10.1302/0301-620x.83b1.10062.
Sousa SR, Barbosa MA. Effect of hydroxyapatite thickness on metal ion release from Ti6Al4 V substrates. Biomaterials. 1996;17(4):397–404. doi:10.1016/0142-9612(96)89655-4.
Bohner M, Lemaitre J. Can bioactivity be tested in vitro with SBF solution Biomaterials. 2009;30:2175–9.
Combes C, Rey C. Adsorption of proteins and calcium phosphate materials bioactivity. Biomaterials. 2002;23:2817–23.
Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–15.
Liu X, Fu RKY, Poon RWY, Chen P, Chu PK, Ding C. Biomimetic growth of apatite on hydrogen-implanted silicon. Biomaterials. 2004;25(25):5575–81. doi:10.1016/j.biomaterials.2004.01.015.
Jaatinen J, Korhonen R, Pelttari A, Helminen H, Korhonen H, Lappalainen R, et al. Early bone growth on the surface of titanium implants in rat femur is enhanced by an amorphous diamond coating. Acta Orthop. 2011;82(4):499–503.
Papo MJ, Catledge SA, Vohra YK. Mechanical wear behavior of nanocrystalline and multilayered diamond coatings on temporomandibular joint implants. J Mat Sci-Mat In Medicine. 2004;15:773.
Fries M, Vohra Y. Nanostructured diamond film deposition on curved surfaces of metallic temporomandibular joint implant. J Phys D Appl Phys. 2002;35(20):L105–7.
Guglielmotti MB, Renou S, Cabrini RL. A histomorphometric study of tissue interface by laminar implant test in rats. Int J Oral Maxillofac Implants. 1999;14(4):565–70.
Booth L, Catledge S, Nolen D, Thompson R, Vohra Y. Synthesis and characterization of multilayered diamond coatings for biomedical implants. Materials. 2011;4:857–68.
Jozwik K, Karczemska A. The new generation Ti6Al4 V artificial heart valve with nanocrystalline diamond coating on the ring and with Derlin disc after long-term mechanical fatigue examination. Diam Relat Mater. 2007;16:1004.
Aspenberg P, Anttila A, Konttinen YT, Lappalainen R, Goodman SB, Nordsletten L, et al. Benign response to particles of diamond and SiC: bone chamber studies of new joint replacement coating materials in rabbits. Biomaterials. 1996;17(8):807–12. doi:10.1016/0142-9612(96)81418-9.
Mattei L, Di Puccio F, Piccigallo B, Ciulli E. Lubrication and wear modelling of artificial hip joints: A review. Tribol Int. 2011;44(5):532–49. doi:10.1016/j.triboint.2010.06.010.
Saikko V, Ahlroos T, Calonius O, Keränen J. Wear simulation of total hip prostheses with polyethylene against CoCr, alumina and diamond-like carbon. Biomaterials. 2001;22(12):1507–14. doi:10.1016/s0142-9612(00)00306-9.
Garrett DJ, Ganesan K, Stacey A, Fox K, Meffin H, Prawer S. Ultra-nanocrystalline diamond electrodes: Optimisation for neural Stimulation. J Neural Eng. 2012;9(1):10.
Aharonovich I, Castelletto S. Simpson D A, Su C-H, Greentree A D, S P. Diamond-based single-photon emitters. Rep Prog Phys. 2011;74(7):076501.
Popov C, Kulisch W, Jelinek M, Bock A, Strnad J. Nanocrystalline diamond/amorphous carbon composite films for applications in tribology, optics and biomedicine. Thin Solid Films. 2006;494(1–2):92–7. doi:10.1016/j.tsf.2005.07.163.
Popov C, Kulisch W, Reithmaier JP, Dostalova T, Jelinek M, Anspach N, et al. Bioproperties of nanocrystalline diamond/amorphous carbon composite films. Diam Relat Mater. 2007;16(4–7):735–9. doi:10.1016/j.diamond.2006.12.001.
Barrere F, van Blitterswijk CA, de Groot K, Layrolle P. Influence of ionic strength and carbonate on the Ca–P coating formation from SBF × 5 solution. Biomaterials. 2002;23(9):1921–30. doi:10.1016/s0142-9612(01)00318-0.
Faig-Martia J, Gil-Murb FJ. Hydroxyapatite coatings in prosthetic joints. Rev Esp Cir Ortop Traumatol. 2008;52:113–20.
Kulisch W, Popov C, Gilliland D, Ceccone G, Reithmaier JP, Rossi F. UNCD/a-C nanocomposite films for biotechnological applications. Surf Coat Technol. 2011;206(4):667–75. doi:10.1016/j.surfcoat.2011.03.057.
McLeod K, Kumar S, Dutta NK, Smart RS, Voelcker NH, Anderson GI. X-ray photoelectron spectroscopy study of the growth kinetics of biomimetically grown hydroxyapatite thin-film coatings. Appl Surf Sci. 2010;256(23):7178–85. doi:10.1016/j.apsusc.2010.05.047.
Chosa N, Taira M, Saitoh S, Sato N, Araki Y. Characterization of apatite formed on alkaline-heat-treated Ti. J Dent Res. 2004;83(6):465–9.
Ben-Nissan B, Chai CS, Gross KA. Effect of solution ageing on sol-gel hydroxyapatite coatings. Bioceramics, Vol 10. 1997.
Suchanek W, Yoshimura M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J Mater Res. 1998;13(1):94–117.
Dorozhkin SV. Bioceramics of calcium orthophosphates. Biomaterials. 2010;31:1465–85.
Kokubo T, Kim H-M, Kawashita M. Novel bioactive materials with different mechanical properties Biomaterials. 2003;24:2161–75.
Kokubo T, Ito S, Shigematsu M, Sakka S. JMS-. Fatigue and lifetime of bioactive glass-ceramic A-W containing apatite and wollastonite. J Mater Sci. 1987;22:4067–70.
Lu X, Leng Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials. 2005;26(10):1097–108. doi:10.1016/j.biomaterials.2004.05.034.
Peng P, Kumar S, Voelcker NH, Szili E. St.C, Griesser H. Thin calcium phosphate coatings on titanium by electrochemical deposition in modified simulated body fluid. J Biomed Mater Res. 2006;76A(2):347–55.
Tanahashi M, Matsuda T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J Biomed Mater Res. 1997;34(3):305–15.
LeGeros RZ. Fundamentals of hydroxyapatite and related calcium phosphates. In: Basu B, Katti D, Kumar A, editors. Advanced Biomaterials: Fundamentals, processing and applications. Hoboken: Wiley; 2009.
Brown WE, Smith JP, Frazier AW, Lehr JR. Crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature. 1962;196(4859):1050. doi:10.1038/1961050a0.
Chou Y-F, Chiou W-A, Xu Y, Dunn JCY, Wu BM. The effect of pH on the structural evolution of accelerated biomimetic apatite. Biomaterials. 2004;25(22):5323–31. doi:10.1016/j.biomaterials.2003.12.037.
Amin M, Randeniya L, Bendavid A, Martin P. E. P. Amorphous carbonated apatite formation on diamond-like carbon containing titanium oxide. Diam Relat Mater. 2009;18:1139–44.
Acknowledgments
A.D.G. acknowledges the Australian Research Council for financial support (Project No. DP0880466). This work was supported by the University of Melbourne Interdisciplinary Seed Funding scheme. K.F. is financially supported by the Australian Research Council (ARC) through its Special Research Initiative (SRI) in Bionic Vision Science and Technology grant to Bionic Vision Australia (BVA) and by the University of Melbourne Research Collaboration Grant scheme. K.F acknowledges the support of Surgical Design and Manufacture Ltd and Prof. Steven Prawer. The authors wish to acknowledge the facilities, and the scientific and technical assistance, of the Australian Microscopy & Microanalysis Research Facility at the RMIT Microscopy & Microanalysis Facility, at RMIT University and Dr Jiri Cervenka for FIB-SEM assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fox, K., Palamara, J., Judge, R. et al. Diamond as a scaffold for bone growth. J Mater Sci: Mater Med 24, 849–861 (2013). https://doi.org/10.1007/s10856-013-4860-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-4860-2