Abstract
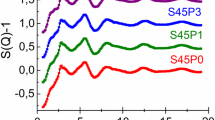
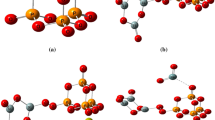
This study was conducted to determine the influence that network modifiers, sodium (Na+) and strontium (Sr2+), have on the solubility of a SiO2–TiO2–CaO–Na2O/SrO bioactive glass. Glass characterization determined each composition had a similar structure, i.e. bridging to non-bridging oxygen ratio determined by X-ray photoelectron spectroscopy. Magic angle spinning nuclear magnetic resonance (MAS-NMR) confirmed structural similarities as each glass presented spectral shifts between −84 and −85 ppm. Differential thermal analysis and hardness testing revealed higher glass transition temperatures (Tg 591–760 °C) and hardness values (2.4–6.1 GPa) for the Sr2+ containing glasses. Additionally the Sr2+ (~250 mg/L) containing glasses displayed much lower ion release rates than the Na+ (~1,200 mg/L) containing glass analogues. With the reduction in ion release there was an associated reduction in solution pH. Cytotoxicity and cell adhesion studies were conducted using MC3T3 Osteoblasts. Each glass did not significantly reduce cell numbers and osteoblasts were found to adhere to each glass surface.










Similar content being viewed by others
References
Jones JR. Review of bioactive glass: from Hench to hybrids. Acta Biomat. 2013;9:4457–86.
Hench LL. The story of bioglass. J Mat Sci. 2006;17:967–78.
Hench LL, Day DE, Holand W, Rheinberger VW. Glass and medicine. Int J App Glass Sci. 2010;1:104–17.
Rahaman M, Day D, Bal B, Fu Q, Jung S, Bonewald L, Tomsia A. Bioactive glass in tissue engineering. Acta Biomat. 2011;6:2235–73.
Chen QZ, Thompson ID, Boccaccini AR. 45S5 Bioglass®-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials. 2006;27:2414–25.
Serra J, Gonzalez P, Liste S, Chiussi S, Leon B, Perez-amor M, Ylanen HO, Hupa M. Influence of the non-bridging oxygen groups on the bioactivity of silicate glasses. J Mat Sci. 2002;13:1221–5.
Serra J, González P, Liste S, Serra C, Chiussi S, León B, Pérez-Amor M, Ylänen HO, Hupa M. FTIR and XPS studies of bioactive silica based glasses. J Non-Crys Sol. 2003;332:20–7.
Branda F, Arcobello-Varlese F, Costantini A, Luciani G. Effect of the substitution of M2O3 (M = La, Y, In, Ga, Al) for CaO on the bioactivity of 2.5CaO-2SiO2 glass. Biomat. 2002;23:711–6.
Shelby JE. Introduction to glass science and technology. 2nd ed. Cambridge: The Royal Society of Chemistry; 2005.
Baker DR. Diffusion of silicon and gallium (as an analogue for aluminum) network-forming cations and their relationship to viscosity in albite melt. Geochim Cosmochim Acta. 1995;59:3561–71.
Mysen BO, Virgo D, Kushiro I. The structural role of aluminium in silicate melts—a Raman spectroscopic study at 1 atmosphere. Amer Mineral. 1981;66:678–701.
Marie PJ. Strontium ranelate: new insights into its dual mode of action. Bone. 2007;40:S5–9.
Marie PJ. Strontium ranelate; a novel mode of action optimizing bone formation and resorption. Osteopor Int. 2005;16:S7–10.
Boyd D, Towler MR, Watts S, Hill R, Wren AW, Clarkin OM. The role of Sr2+ on the structure and reactivity of SrO-CaO-ZnO-SiO2 ionomer glasses. J Mat Sci. 2008;19:953–7.
Aguiar H, Serra J, González P, León B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J Non-Cryst Sol. 2009;355:475–80.
McMillian PW. Structural studies of silicate glasses and melt-applications and limitations of Raman spectroscopy. Am Mineral. 1984;69:622–44.
Hayakawa S, Osaka A, Nishioka H, Matsumoto S, Minura Y. Structure of lead oxyfluorosilicate glasses: X-ray photoelectron spectroscopy and nuclear magnetic resonance spectroscopy and molecular dynamics simulation. J Non-Cryst Sol. 2000;272:103–18.
Galliano PG, Porto JM, Spezl L, Varetti EL, Sobrados I, Sanz J. Analysis by nuclear magnetic resonance and raman spectroscopies of the structure of bioactive alkaline-earth silicophosphate glasses. Mat Res Bull. 1994;29:1297–306.
Murphy S, Wren AW, Towler MR, Boyd D. The effect of ionic dissolution products of Ca–Sr–Na–Zn–Si bioactive glass on in vitro cytocompatibility. J Mat Sci. 2010;10:2827–34.
Hoppe A, Guldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–74.
Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity. Biomat. 2006;27:2907–15.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Placek, L.M., Coughlan, A. et al. Investigating the influence of Na+ and Sr2+ on the structure and solubility of SiO2–TiO2–CaO–Na2O/SrO bioactive glass. J Mater Sci: Mater Med 26, 85 (2015). https://doi.org/10.1007/s10856-015-5415-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5415-5