Abstract
Purpose
There is still scarce data on SARS-CoV-2 infection in patients with Inborn Errors of Immunity (IEI) and many unresolved questions. We aimed to describe the clinical outcome of SARS-CoV-2 infection in Brazilian IEI patients and identify factors influencing the infection.
Methods
We did a cross-sectional, multicenter study that included patients of any age affected by IEI and SARS-CoV-2 infection. The variables studied were sex, age, type of IEI, comorbidities (number and type), treatment in use for IEI, clinical manifestations and severity of SARS-CoV-2 infection.
Results
121 patients were included: 55.4% female, ages from six months to 74 yo (median age = 25.1 yo). Most patients had predominantly antibody deficiency (n = 53). The infection was mostly asymptomatic (n = 21) and mild (n = 66), and one child had multisystem inflammatory syndrome (MIS-C). We could not observe sex-related susceptibility, and there was a weak correlation between age and severity of infection. The number of comorbidities was higher in severe cases, particularly bronchiectasis and cardiopathy. There were no severe cases in hereditary angioedema patients. Six patients aged 2 to 74 years died, three of them with antibody deficiency.
Conclusion
The outcome was mild in most patients, but the Case Fatality Ratio was higher than in the general population. However, the type of IEI was not a determining factor for severity, except for complement deficiencies linked to milder COVID-19. The severity of SARS-CoV-2 infection seems to be more related to older age, a higher number of comorbidities and type of comorbidities (bronchiectasis and cardiopathy).
Similar content being viewed by others
Introduction
SARS-CoV-2 infections emerged by the end of 2019, and since March 2020, we have experienced a pandemic. Most people (80%) with COVID-19 have mild respiratory symptoms [1], particularly children that, in general, are asymptomatic or have mild symptoms [2]. The disease includes severe pulmonary involvement in approximately 14% of individuals, while critical illness involving respiratory failure, sepsis, or multiple organ failure affects 5% of patients [1]. The case fatality ratio (CFR) varies from 1 to 5% in most countries, and in Brazil, it is around 2.5% [3].
Risk factors for severe COVID-19 include old age and a few medical conditions: cancer, chronic kidney disease, chronic obstructive pulmonary disease (COPD), Down syndrome, heart conditions, obesity, pregnancy, sickle cell disease, solid organ transplantation, smoking, and type 2 diabetes mellitus [4].
Inborn errors of immunity (IEI) or primary immunodeficiencies (PID) are a heterogeneous group of diseases including more than 430 different gene defects [5] that could relate to a lower or a higher risk of complications by SARS-CoV-2 infection. According to the identified immunological defect, a group of experts from the UK developed a document classifying the risk of severe infection by SARS-CoV-2. This document was based on previous knowledge about the behavior of various immunological defects toward viral infections [6]. However, the clinical experience with SARS-CoV-2 infections was just starting.
Case reports and case series have been published about IEI patients’ clinical outcomes [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. Still, there are little data reported on SARS-CoV-2 in IEI patients and many unanswered questions.
Brazil is the third country in the world in the number of cases of COVID-19 and the second in the number of deaths and has health policies and socio-demographic characteristics differing from other countries reporting about patients with IEI and SARS-CoV-2 infection [22]. In this study we propose to describe the clinical development of SARS-CoV-2 infection in IEI Brazilian patients and to identify factors that influence the outcome.
Methods
We developed a cross-sectional, multicenter study, following STROBE statement including reference centers for IEI and hematopoietic stem cell transplantation (HSCT). The inclusion criteria were patients of any age diagnosed with an IEI according to the criteria used by the ESID registry [23] and confirmed diagnosis of SARS-CoV-2 infection by PCR and/or serology and/or point-of-care tests or presenting typical symptoms and/or imaging tests and history of contact with a confirmed case of SARS-CoV-2 infection. Patients with typical clinical manifestations or manifestations compatible with SARS-CoV-2 infection who did not have typical imaging and/or history of contact with confirmed cases of infection by this virus were excluded.
The following variables were assessed: sex, age, type of IEI, comorbidities (number and type), and the treatment in use before SARS-CoV-2 infection diagnosis and clinical manifestations of the infection. The severity of SARS-CoV-2 infection was defined by the researchers, following NIH criteria [24], as asymptomatic, mild, moderate, severe, and critical. Type of IEI was defined according to the 2019 IUIS classification tables [5]. Case fatality ratio was calculated according to WHO scientific brief [25].
The data were inserted in IBM® SPSS Statistics (version 26) spreadsheet. Descriptive and statistical inference tools were used to analyze the correlation between categorical and numerical variables, with a significant p value ≤ 0.05. We performed correlations between variables: sex, median age, group of IEI, number and type of comorbidities, medications in use before infection × severity of SARS-CoV-2 infection; the number of comorbidities × age group; and type of clinical manifestation × severity of COVID-19 in different age groups. For correlation between ordinal variables, we used Kendall tau-b correlation coefficient and Gamma measure of association. For correlation between nominal/ordinal variables, we used Lambda measure of association and Cramer’s V coefficient. Whenever possible, Fisher’s test was used to assess significance. When not possible (by limitations of the software used), the Monte Carlo method was applied. Kruskal–Wallis test was used to compare numerical variables with different levels of SARS-CoV-2 infection severity.
The project was approved by the Research Ethics Committee (CAAE 31,264,220.0.1001.5264). Patients and/or their relatives signed a consent form to participate in the study.
Results
We collected data from 121 patients diagnosed with IEI and infected by SARS-CoV-2 between March and December 2020. RT-PCR tests confirmed the diagnosis of SARS-CoV-2 infection from nasopharyngeal or nasal swab in 82 patients (67.8%); using RT-PCR and serology, in three patients (2.5%); using serology only in 22 patients (18.2%); and only by point-of-care test, in three patients (2.5%), totaling 110 (90.9%) patients. The other 11 patients (9.1%) were included since they presented typical clinical manifestations of SARS-CoV-2 infection and/or typical imaging tests and history of contact with confirmed cases. No reinfections were reported during the study period.
Patients’ ages ranged from 6 months to 74 years old (median age of 25.1 years). Age distribution was: ≤ 18 years old, 57 patients (47.1%); 19–59 years old, 60 patients (49.6%); and ≥ 60 years old, four (3.3%) patients. Sixty-seven patients were female (55.4%). Thirteen patients with hereditary angioedema were included in Grumach et al. [26], four patients (two with familial Mediterranean fever and two with CVID) were already reported in Meyts’s study [14], and another CVID patient was previously reported [20] (these patients are highlighted in the supplementary material).
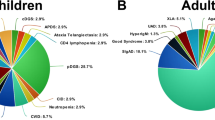
Most patients were diagnosed with predominantly antibody deficiencies (n = 53/43.8%) and complement deficiencies (n = 25/20.7%). The distribution of cases according to the IEI classification group is shown in Fig. 1A.
A Distribution of IEI groups composed of 121 patients with a diagnosis of SARS-CoV-2 infection. Predominantly antibody deficiencies n = 53; complement deficiencies n = 25; combined T and B cell deficiencies n = 12 (seven of them transplanted); autoinflammatory diseases n = 9; innate immunity defects n = 7, phagocyte defects n = 6 (one of them transplanted); combined T and B cell deficiencies associated with syndromes n = 5 (one of them transplanted); immune dysregulation diseases n = 3 (one of them transplanted); bone marrow failure n = 0; and phenocopies of immunodeficiencies n = 1. B Severity of acute SARS-CoV-2 infection in 120 patients: asymptomatic n = 21; mild n = 66, moderate n = 11, severe n = 16, critical n = 6. Def – deficiencies; SCID – severe combined immunodeficiency; CID – combined immunodeficiency; Ab – antibody; Imm – immune; Dysreg – Dysregulation; Dis – diseases; Autoinflam – autoinflammatory
Before SARS-CoV-2 infection, 49/121 (40.5%) patients were under regular human immunoglobulin (IG) replacement, either intravenously (89.8%) or subcutaneously (10.2%). Chemoprophylaxis was used by 31/121 (25.6%) patients, and 11/121 (9.1%) were using azithromycin. The use of immunosuppressors was reported in 11.6% (n = 14), one of them for the hemophagocytic syndrome, the other for lymphoma, and the rest for autoimmune disorders. Other drugs included androgens in 8.3% (n = 10) and “immunomodulators” (colchicine or hydroxychloroquine) in 6.8% (n = 8).
Comorbidities were absent in 70 patients (57.8%); at least one comorbidity was observed in 37 patients (30.5%); two comorbidities in 10 patients (8.3%); and three or more comorbidities in four (3.3%). Lung diseases were the most frequent comorbidities (n = 34): 15.7% presented bronchiectasis (n = 19); 7.4%, asthma (n = 9); and 5%, other lung diseases (n = 6). Systemic arterial hypertension affected 11.6% (n = 14); obesity, 8.3% (n = 10); overweight, 1.7% (n = 2); gastrointestinal disorders, 5.7% (n = 8); surgically corrected congenital heart diseases, 2.5% (n = 3); Down syndrome, 2.5% (n = 3); type II diabetes mellitus, 3.3% (n = 4); and type I diabetes mellitus, 1.7% (n = 2). One patient was pregnant.
A hundred and twenty IEI patients presented acute SARS-CoV-2 infection where 21 were classified as asymptomatic and 99 were symptomatic (i.e., had COVID-19) (Fig. 1B). One patient presented MIS-C. From 100 symptomatic patients (99 diagnosed with COVID-19 and one patient with MIS-C), 35 patients were hospitalized and 94 recovered. The 21 asymptomatic patients underwent the SARS-CoV-2 RT-PCR test either because of hospitalization for another reason than COVID-19 or due to a history of contact with a positive case.
There was no correlation between sex and severity of the infection, neither in general (Kendall’s tau-b 0.095, p = 0.265 Fisher’s test) nor in the group of children/adolescents (Kendall’s tau-b 0.052, p = 0.693 Fisher’s test) or the group of adults (Kendall’s tau-b 0.032, p = 0.794 Fisher’s test).
A significant difference in the median age among the five levels of severity of SARS-CoV-2 infection was identified (Kruskal–Wallis test p = 0.004). The asymptomatic group median age was lower than for the other levels of severity. However, a significant difference was detected between asymptomatic and mild cases and also severe cases but not between asymptomatic and critical or moderate cases (age standard deviation for critical and moderate cases was higher). No significant difference was identified between mild, moderate, severe, and critical cases median ages (Fig. 2).
A clear correlation between the IEI group and the severity of infection was not identified (Gamma 0.001, p = 0.992; Kendall’s tau-b 0.001, p = 0.992 Monte Carlo method). Complement deficiencies were the second most common IEI, and no report of severe cases was observed. On the other hand, in the phenocopy group, a single patient reported died. The distribution of SARS-CoV-2 infections severity in the different types of IEI is described in Fig. 3.
Severity of SARS-CoV-2 infection in 120 patients with various IEI (excluding patient with MIS-C). SCID – severe combined immunodeficiency (seven post-HSCT); CID – combined immunodeficiency; WAS – Wiskott-Aldrich syndrome (one post-HSCT); CVID – common variable immunodeficiency; XLA – X-linked agammaglobulinemia; APDS – activated p110δ Syndrome (PI3KCD); FHL – familial hemophagocytic lymphohistiocytosis; CHS – Chediak-Higashi syndrome; XIAP – one post-HSCT; CGD – chronic granulomatous disease (three males, probably XL form, and one female CYBA); LAD – leukocyte adhesion deficiency (one post- HSCT); MSMD – Mendelian susceptibility to mycobacterial infections (one IFNGR1, one IFNGR2); WHIM – warts, hypogammaglobulinemia, infections, myelokathexis; CMC – chronic mucocutaneous candidiasis (probably IL-17 defect); FMF – familiar Mediterranean fever; AID – autoinflammatory disease; CAPS – cryopyrin-associated periodic syndrome; HAE – hereditary angioedema
There was a weak correlation between the use of immunoglobulin and the higher severity of SARS-CoV-2 infection (Cramer’s V 0.345, p = 0.005 Fisher’s test) and between the use of immunosuppressors and lower severity of SARS-CoV-2 infection (Cramer’s V 0.304, p = 0.029 Fisher’s test). Six/14 patients who were in use of immunosuppressors were asymptomatic, 4/14 had mild COVID-19, and one had a critical COVID-19.
A difference in the number of comorbidities concerning the severity of SARS-CoV-2 infection was observed (Kruskal–Wallis test p < 0.001), but there was a difference only between asymptomatic + mild + moderate cases and severe + critical cases. In addition, there was a difference between the number of comorbidities and the age group of patients (Kruskal–Wallis test p = 0.008), but only between ≤ 18-year-old + 19–59-year-old groups and ≥ 60 years old, but not between ≤ 18 years old and 19–59-year-old groups.
The correlation between types of comorbidities presented by the patients and the severity of SARS-CoV-2 infection showed a weak correlation between the presence of bronchiectasis (Cramer’s V 0.365, p = 0.004 Fisher’s test) and cardiopathy (Cramer’s V 0.302, p = 0.047 Fisher’s test) and higher severity. The pregnant patient, who had an autoinflammatory disease (CAPS-NLRP12 mutation) presented as mild COVID-19.
Fever was the most common symptom, reported in 66/99 (66.7%) patients with COVID-19. Other frequent symptoms were cough in 56.6% (n = 56), upper airway symptoms (sore throat, nasal congestion, coryza) in 53.3% (n = 53), hypo- or anosmia in 38.4% (n = 380, headache in 32.2% (n = 32), dyspnea in 29.3% (n = 29), dys- or ageusia in 29.3% (n = 29), diarrhea in 23.2% (n = 23), fatigue in 8.1% (n = 8), chest pain in 6.1% (n = 6), abdominal pain in 5.1% (n = 5), vomiting in 4% (n = 4), and pericarditis in 2% (n = 2). Other clinical manifestations have been described in only one case each: conjunctivitis, ocular and nasal burning, parotitis, cholestasis, and oral ulcers. Five patients with hereditary angioedema (35.7% of the 24 patients) presented edema attacks: four with subcutaneous edema (one also with abdominal crisis) and one with laryngeal edema. Five patients presented cutaneous rash and one of them had a previous diagnosis of CAPS-NLRP3 and presented a diffuse cutaneous maculopapular rash that on skin biopsy demonstrated lymphocytic vasculitis, probably related to the viral infection. Bacterial pneumonia was a secondary diagnosis in 11 cases and sepsis in two, but with no identification of an infectious agent.
A 6-year-old girl with a possible innate immunity defect with susceptibility to bacterial infections (genetic testing in progress) presented fever, skin rash, pleural and pericardial effusion, ascites, and nonspecific pulmonary infiltrate with dragged evolution. SARS-CoV-2 RT-PCR by swab was three times negative, and serology (IgG > 100 AU/mL) was also positive three times. In addition, cytokine dosage showed a significant increase of IL-10 and a lower increase in IL-6, diagnosed as a systemic inflammatory condition after infection by SARS-CoV-2 (MIS-C).
The correlation between clinical manifestations and severity of COVID-19 in patients ≤ 18 years showed a moderate correlation between rash (Cramer’s V 0.598, p = 0.024 Fisher’s test) and vomiting (Cramer’s V 0.598, p = 0.024 Fisher’s test) with higher severity. These symptoms were identified only in the ≤ 18-year-old group. Clinical manifestations and severity of COVID-19 in patients ≥ 19 years old showed a correlation between cough (weak, Cramer’s V 0.337, p = 0.027 Fisher’s test) and dyspnea (moderate-Cramer’s V 0.456, p = 0.003 Fisher’s test) both associated with higher severity.
Ten patients with SCID (n = 7), LAD type III (n = 1), WAS (n = 1), and XIAP mutation (n = 1) had SARS-CoV-2 infection after hematopoietic stem cell transplant (HSCT) (see Table 1). The majority (n = 7) were detected after 100 days of transplantation, and of these, four were asymptomatic and three had mild COVID-19. Among the three patients with SARS-CoV-2 infection within the first 100 days of HSCT, two were asymptomatic. A third one was a patient with XIAP mutation presenting mild COVID-19 symptoms (fever and rash with positive RT-PCR) on the sixth day after a haploidentical HSCT. He developed multiple complications related to poor graft function and veno-occlusive disease and died due to a fungal infection four months after being hospitalized most of the time.
Patients classified as critical COVID-19 died. All of them presented severe pulmonary manifestation with sepsis and/or multiple organ failure. Four were male, ranging from two to 74 years, with a median age of 20.2 years. Two patients were diagnosed with X-linked agammaglobulinemia, one with common variable immunodeficiency, one with hyper IgM syndrome (CD40L defect), and one with Good syndrome.
The patient with hyper IgM syndrome was a 15-year-old boy with no comorbidities and a severe inflammatory manifestation of COVID-19. One of the deceased patients with a diagnosis of X-linked agammaglobulinemia was an obese child. The other patient with X-linked agammaglobulinemia was a young adult with bronchiectasis, asthma, hypertension, and overweight. A year younger, this patient’s brother, also diagnosed with X-linked agammaglobulinemia, obese, and no other comorbidities, evolved with COVID-19 classified as moderate and recovered well. The deceased patient with common variable immunodeficiency presented bronchiectasis and arterial hypertension. The patient with Good syndrome (74 years old) who had myasthenia gravis went through adrenalectomy and pulmonary lobectomy previously.
Case fatality ratio and inpatient mortality were 5% and 17.1%, respectively, among all 121 patients. CFR and inpatient mortality according to IEI classification were, respectively: antibody deficiencies (n = 53) 6.38% and 17.6%; non-severe combined T/B cells deficiency (n = 5) 20% and 50%, immune dysregulation (n = 3) 50% and 50%; and phenocopies (n = 1) 100% and 100%. CFR in ≤ 18-year-old group (n = 57) was 3.5%; in 19–59 years old (n = 60), 5% and in ≥ 60 years old (n = 4), 25%.
Data from the 121 patients of the study are detailed in supplemental file 1.
Discussion
The restricted knowledge about the immunopathology of SARS-CoV-2 infection for IEI patients raised the question about the clinical impact of COVID-19 and the risk involved for these patients [27]. As already mentioned, several case reports were previously published [7,8,9,10,11,12,13, 15], as well as two large series: 94 IEI patients in Meyts et al. study, from ESID [14], and 60 IEI patients and 33 secondary immunodeficient patients from Shields et al. study [16], in the UK.
In our group of 121 IEI patients with SARS-CoV-2 infection, females were more frequent (55.4%), unlike Meyts et al. and Marcus et al.’s studies [14, 21], however similar to the 60 IEI patients from Shields et al. [16] report. Our group of patients was younger than the other series of patients.
Predominantly antibody deficiencies represented the most frequent IEI among our patients (43.8%) as described in other series [14, 16, 21], but in a lower proportion, particularly in comparison to Shields’ group (76.7%). In our series, the second most frequent IEI was complement deficiencies (20.7%), absent from Meyts’s group and in lower numbers in Shields’ group (6.7%), due to different structured groups of specialists registering HAE patients.
Most of our patients had no comorbidities, unlike Meyts’s and Shields’ samples, mainly having lung disease comorbidity. This characteristic is probably related to the younger age of our patients. Most of our patients were symptomatic (n = 100; 82.6%). In particular, Brazil has not implemented mass testing, and the SARS-CoV-2 tests are usually performed in hospitalized patients, especially in the public health system. Since patients with IEI have not been routinely tested or tested whenever they had a history of contact or symptoms, it is impossible to affirm that patients with IEI have more frequent symptoms related to SARS-CoV-2 infection than the general population. We have a younger group of patients, and, still, the percentage of asymptomatic patients (17.4%) was slightly above that presented in the ESID series (11%) [14]. In addition, most symptomatic patients presented mild cases of COVID-19, as reported by Meyts et al. [14] and by Marcus et al. [21]. The study by Marcus et al. presented a series of 20 patients between 4 months and 60 years of age, 60% male, 80% having alterations in the production of antibodies, and seven of them with combined defects of T and B cells (one of them 20 years after hematopoietic stem cell transplantation). No deaths were reported, none of them required hospitalization because of SARS-CoV-2 infection, and seven of them (including one 22q11.2 deletion, one CGD, two combined immunodeficiency, one XLA, and one CVID) were asymptomatic. [21]
The symptoms most frequently described in our 99 patients with COVID-19 were similar to those described in other series of patients with or without IEI [14, 28,29,30,31]. Unlike published data, we have not identified headache, olfactory disorders, or taste disorders as markers of milder COVID-19 [32, 33]. Cough and dyspnea have been described before as predictors of severity in adults [34,35,36]. However, cutaneous rash and vomiting have not been described before as predictors of severity in children [37,38,39].
We report one case with a possible innate immunity defect with susceptibility to bacterial infections and MIS-C diagnosis based on previously reported differences in relation to the inflammatory phase of COVID-19 [40]. There are two previous MIS-C reports in patients with IEI: a patient with SOCS1 haploinsufficiency, a disease characterized by early-onset autoimmunity due to reduced JAK-STAT pathway inhibition [41] and a patient with IFNGR2 defect, reported in Meyts et al. study [14]. Another patient in our series, with confirmed IRAK4 deficiency, presented a severe inflammatory condition related to COVID-19, ratifying the role of innate immunity defects in the pathogenesis of severe SARS-CoV-2 infection [42].
We observed no sex susceptibility difference for severe SARS-CoV-2 infections in the group ≥ 19 years old nor in the ≤ 18-year-old group. No sex difference for COVID-19 susceptibility has been observed in children [43], although it is frequently reported in adults [44, 45]. It is important to note that HAE was the second most common IEI in our group of patients, more frequent in females due to hormones’ influence and related to less severe form of COVID-19. Moreover, we found no male predominance for severe cases.
Young patients had severe cases in our series. Also, we did not identify protection against severe COVID-19 among patients with antibody deficiencies, as was shown by Meyts’s [14], Ho’s [12], and Shields’s [16], and contrary to what was reported at the beginning of the pandemic [17, 46] and by Marcus et al. [21]. Five (83.3%) deceased patients were ≤ 59 years old, and three (50%) of them had antibody deficiencies. The results of our analyses suggest that the presence of comorbidities, particularly the presence of bronchiectasis, is responsible for this finding. In addition, we can hypothesize that the production of antibodies is not irrelevant in the immune response to SARS-CoV-2. It is essential to report that only one of our patients (CVID, previously published) received convalescent plasma (not widely available in Brazil), described as effective in some patients with antibody deficiencies [13, 20, 47]. We ratified findings from previous publications [26, 48] on the prevalence of mild conditions among patients with complement deficiencies, and more than a third presented edema attacks. Edema attacks in 31% of HAE patients during SARS-CoV-2 infections have also been described recently, but with two cases of severe infections [49].
The outcome of SARS-CoV-2 infections was less severe in patients submitted to HSCT in our group, particularly those having the disease more than 100 days post-transplantation, as it was expected. However, even the patients in our series who had the infection before 100 days post-transplantation had better outcomes than reported in a group of 318 HSCT recipients, including only four IEI patients [50].
The use of immunoglobulin was related to severe SARS-CoV-2 infection. It is important to note that 14/19 patients with bronchiectasis received IG, a possible explanation for this finding. On the other hand, immunosuppressors, a risk factor reported in some publications [4, 27] and a non-interfering factor in others [51], were identified by our group as a possible protective factor against severe cases, probably by reducing the inflammatory process. The use of androgens was not related to higher severity, suggesting that male hormones’ proposed interference in higher severity of COVID-19 should be further investigated [52].
A correlation between the number of comorbidities and increased severity of COVID-19 was detected, but not between < 18-year-old and 19–59-year-old groups. In addition, we had only four patients in this oldest group. Probably that is the reason we have not identified a strong correlation between age and severity. The presence of bronchiectasis and cardiopathy showed a correlation with higher severity of SARS-CoV-2 infection, as shown by Shields et al. [16]. These observations suggest that comorbidities are a determinant factor for SARS-CoV-2 infection severity in our series of patients.
We registered 6 deaths out of 121 cases, a lower percentage (5%) than the 10% reported by Meyts et al. [14], probably because our group is younger, with fewer comorbidities and a female predominance. CFR in our group of antibody deficiency patients (6.38%) is much lower than in Ho et al. [12] series (25%), composed of adults only and predominantly males. CFR (5%) and inpatient mortality (17.1%) in our group were also considerably lower than the values reported by Shields et al. [16] (31.6% and 37.5%, respectively), most likely due to the lower age of our patients and fewer comorbidities, as both series of patients were female-biased. However, our CFR values (5%) are considerably higher than those described in Brazil’s general population (2.5%) [3].
Our study has some limitations. Asymptomatic patients could not be detected as there was no systematic testing in our population. Also, the under-representation of several IEI diseases in our series and the low number of patients and study’s descriptive methodology compromise the statistical analysis, preventing, therefore, precise statements about the relationship between the severity of COVID-19, all types of IEI and other variables. The pandemic’s evolution and the increasing number of IEI patients infected by SARS-CoV-2 may change our punctual observation. Nevertheless, until now, the series reported here represents one of the largest studying IEI and SARS-CoV-2 infection.
In conclusion, the disease outcome was mild in most patients with IEI, but CFR was higher than in the general population. Regarding HAE, SARS-CoV-2 represents a trigger factor for edema attacks. The severity of SARS-CoV-2 infection in IEI patients seems to be related to older age, especially to a higher number of comorbidities and types of comorbidities (bronchiectasis and cardiopathy). We do not know how the pandemic’s evolution will develop, despite the enormous expectation about the start of active immunization. Therefore, we consider it relevant to keep studying different aspects of SARS-CoV-2 infection in patients with IEI.
Data Availability
Data of all patients are described in the supplementary excel file.
Code Availability
IBM® SPSS Statistics (version 26).
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- CFR:
-
Case fatality ratio
- COPD:
-
Chronic obstructive pulmonary disease
- CVID:
-
Common variable immunodeficiency
- ESID:
-
European Society for Immunodeficiencies
- HAE:
-
Hereditary angioedema
- HSCT:
-
Hematopoietic stem cell transplant
- IEI:
-
Inborn errors of immunity
- IFR:
-
Infection fatality ratio
- IG:
-
Immunoglobulin
- IUIS:
-
International Union of Immunological Societies
- MIS-C:
-
Multisystem inflammatory syndrome in children
- NIH:
-
National Institutes of Health
- PID:
-
Primary immunodeficiencies
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- UK:
-
United Kingdom
References
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42.
Bixler D, Miller AD, Mattison CP, Taylor B, Komatsu K, Peterson Pompa X, et al. SARS-CoV-2-associated deaths among persons aged <21 years - United States, February 12-July 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1324–9.
Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–4.
National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. People with Certain Medical Conditions. CDC; 2020 [updated Dec. 29, 2020]. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fgroups-at-higher-risk.html. Accessed 15 Jan 2020.
Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40(1):24–64.
United Kingdom Primary Immunodeficiency Network (UKPIN). Advice for healthcare professionals looking after patients with immunodeficiency regarding COVID-19. UKPIN; 2020 [updated March 24, 2020]. Available at: https://www.ukpin.org.uk/docs/default-source/default-document-library/ukpin_risk_stratification_covid19_finalac6baa9cd4eb6fe9b40eff00005026c1.pdf Accessed 15 Jan 2020.
Ahanchian H, Moazzen N, Faroughi MSD, Khalighi N, Khoshkhui M, Aelami MH, et al. COVID-19 in a child with primary specific antibody deficiency. Research Square.2020, https://onlinelibrary.wiley.com/doi/pdf/https://doi.org/10.1002/ccr3.3643
Aljaberi R, Wishah K. Positive outcome in a patient with coronavirus disease 2019 and common variable immunodeficiency after intravenous immunoglobulin. Ann Allergy Asthma Immunol. 2020;125(3):349–50.
Castano-Jaramillo LM, Yamazaki-Nakashimada MA, Scheffler Mendoza SC, Bustamante-Ogando JC, Espinosa-Padilla SE, Lugo Reyes SO. A male infant with COVID-19 in the context of ARPC1B deficiency. Pediatr Allergy Immunol. 2021;32(1):199–201.
Cohen B, Rubinstein R, Gans MD, Deng L, Eisenberg R, Rubinstein A. COVID-19 Infection in ten common variable immunodeficiency patients in New York city. J Allergy Clin Immunol In Practice. 2021;9(1):504-507.e1.
Fill L, Hadney L, Graven K, Persaud R, Hostoffer R. The clinical observation of a patient with common variable immunodeficiency diagnosed as having coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020;125(1):112–4.
Ho HE, Mathew S, Peluso MJ, Cunningham-Rundles C. Clinical outcomes and features of COVID-19 in patients with primary immunodeficiencies in New York City. J Allergy Clin Immunol In Practice. 2021;9(1):490-493.e2.
Jin H, Reed JC, Liu STH, Ho HE, Lopes JP, Ramsey NB, et al. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J Allergy Clin Immunol Pract. 2020;8(10):3594-3596 e3.
Meyts I, Bucciol G, Quinti I, Neven B, Fischer A, Seoane E, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2020. https://doi.org/10.1016/j.jaci.2020.09.010.
Mullur J, Wang A, Feldweg A. A fatal case of coronavirus disease 2019 in a patient with common variable immunodeficiency. Ann Allergy Asthma Immunol. 2021;126(1):90–2.
Shields AM, Burns SO, Savic S, Richter AG, consortium UPC-. COVID-19 in patients with primary and secondary immunodeficiency: the United Kingdom experience. J Allergy Clin Immunol. 2020. https://doi.org/10.1016/j.jaci.2020.12.620
Soresina A, Moratto D, Chiarini M, Paolillo C, Baresi G, Foca E, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol. 2020;31(5):565–9.
Van Damme KFA, Tavernier S, Van Roy N, De Leeuw E, Declercq J, Bosteels C, et al. Case report: convalescent plasma, a targeted therapy for patients with CVIDD and severe COVID-19. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.596761.
Fallatah E, Chang Y, Calderon J, Trujillo VH. DiGeorge syndrome and COVID-19 in two pediatric patients. J Allergy Clin Immunol. 2021;147(2):AB66.
Ribeiro LC, Benites BD, Ulaf RG, Nunes TA, Costa-Lima C, Addas-Carvalho M, et al. Rapid clinical recovery of a SARS-CoV-2 infected common variable immunodeficiency patient following the infusion of COVID-19 convalescent plasma. Allergy Asthma Clin Immunol. 2021;17(1):14.
Marcus N, Frizinsky S, Hagin D, Ovadia A, Hanna S, Farkash M, et al. Minor clinical impact of COVID-19 pandemic on patients with primary immunodeficiency in Israel. Front Immunol. 2020;11:614086.
World Health Organization. WHO coronavirus disease (COVID-19) Dashboard (web). https://covid19.who.int: WHO; 2020. Available at https://covid19.who.int/ Accessed Jan15 2020.
Seidel MG, Kindle G, Gathmann B, Quinti I, Buckland M, van Montfrans J, et al. The European Society for Immunodeficiencies (ESID) registry working definitions for the clinical diagnosis of inborn errors of Immunity. J Allergy Clin Immunol In Practice. 2019;7(6):1763–70.
National Institutes of Heakth (NIH). Clinical spectrum of SARS-CoV-2 infection [updated Dec 17 2020]. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Accessed 15 Jan 2020.
World Health Organization. Estimating mortality from COVID-19. 2020. Report No.: WHO-2019-nCoV-Sci_Brief-Mortality-2020.1. Available at: https://apps.who.int/iris/bitstream/handle/10665/333642/WHO-2019-nCoV-Sci_Brief-Mortality-2020.1-eng.pdf?sequence=1&isAllowed=y Accessed 15 Jan 2020.
Grumach AS, Goudouris E, Dortas Junior S, Marcelino FC, Alonso MLO, Martins RO, et al. COVID-19 affecting hereditary angioedema patients with and without C1 inhibitor deficiency. J Allergy Clin Immunol Pract. 2021;9(1):508–10.
Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):e93–5.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis (Berl). 2020;7(2):91–6.
de Souza WM, Buss LF, Candido DDS, Carrera JP, Li S, Zarebski AE, et al. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nat Hum Behav. 2020;4(8):856–65.
Teich VD, Klajner S, Almeida FAS, Dantas ACB, Laselva CR, Torritesi MG, et al. Epidemiologic and clinical features of patients with COVID-19 in Brazil. Einstein (Sao Paulo). 2020;18:eAO6022.
Gonzalez-Martinez A, Fanjul V, Ramos C, Serrano Ballesteros J, Bustamante M, Villa Marti A, et al. Headache during SARS-CoV-2 infection as an early symptom associated with a more benign course of disease: a case-control study. Eur J Neurol. 2021. https://doi.org/10.1111/ene.14718.
Sedaghat AR, Gengler I, Speth MM. Olfactory dysfunction: a highly prevalent symptom of COVID-19 with public health significance. Otolaryngol Head Neck Surg. 2020;163(1):12–5.
Barek MA, Aziz MA, Islam MS. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: a meta-analysis with 55 studies and 10014 cases. Heliyon. 2020. https://doi.org/10.1016/j.heliyon.2020.e05684.
Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65(5):533–46.
Zhang SY, Lian JS, Hu JH, Zhang XL, Lu YF, Cai H, et al. Clinical characteristics of different subtypes and risk factors for the severity of illness in patients with COVID-19 in Zhejiang, China. Infect Dis Poverty. 2020, https://idpjournal.biomedcentral.com/articles/https://doi.org/10.1186/s40249-020-00710-6
Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children: a systematic review. Pediatr Pulmonol. 2020;55(10):2565–75.
Du H, Dong X, Zhang JJ, Cao YY, Akdis M, Huang PQ, et al. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy. 2020. https://doi.org/10.1111/all.14452.
Ghazal S, Litvinov IV, Aljahani N, Jfri A, Netchiporouk E. Cutaneous manifestations of coronavirus disease 2019 (COVID-19) infection-what do we know so far? J Cutan Med Surg. 2020;24(4):416–7.
Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130(11):5967–75.
Lee PY, Platt CD, Weeks S, Grace RF, Maher G, Gauthier K, et al. Immune dysregulation and multisystem inflammatory syndrome in children (MIS-C) in individuals with haploinsufficiency of SOCS1. J Allergy Clin Immunol. 2020;146(5):1194–200 e1.
Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4570.
Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020. https://doi.org/10.1542/peds.2020-0702.
Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. https://doi.org/10.3389/fpubh.2020.00152.
Lakbar I, Luque-Paz D, Mege JL, Einav S, Leone M. COVID-19 gender susceptibility and outcomes: a systematic review. PLoS ONE. 2020;15(11):e0241827. https://doi.org/10.1371/journal.pone.0241827.
Quinti I, Lougaris V, Milito C, Cinetto F, Pecoraro A, Mezzaroma I, et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146(1):211-213 e4.
Milosevic I, Jovanovic J, Stevanovic O. Atypical course of COVID-19 in patient with Bruton agammaglobulinemia. J Infect Dev Ctries. 2020;14(11):1248–51.
AŞIk A, Mete GÖKmen N. COVID-19 Disease and hereditary angioedema. Asthma Allergy Immunology. 2020.
Belbezier A, Arnaud M, Boccon-Gibod I, Pelletier F, McAvoy C, Gobert D, et al. COVID-19 as a trigger of acute attacks in people with hereditary angioedema. Clin Exp Allergy. 2021.
Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021. https://doi.org/10.1016/S2352-3026[20]30429-4.
Andersen KM, Mehta HB, Palamuttam N, Ford D, Garibaldi BT, Auwaerter PG, et al. Association between chronic use of immunosuppresive drugs and clinical outcomes from coronavirus disease 2019 (COVID-19) hospitalization: a retrospective cohort study in a large US health system. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciaa1488.
Foresta C, Rocca MS, Di Nisio A. Gender susceptibility to COVID-19: a review of the putative role of sex hormones and X chromosome. J Endocrinol Invest. 2020, https://link.springer.com/article/10.1007%2Fs40618-020-01383-6
Acknowledgements
The authors would like to acknowledge all patients and their families, who allowed the data to be used in this publication, particularly to those who tragically lost their loved ones, and to Jeffrey Modell Foundation (not-for-profit institution) for funding for the preparation of the article.
Funding
This study received Jeffrey Modell Foundation (not-for-profit institution) funding for the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors have contributed substantively and intellectually to this paper. The contributions of each author are described in the cover letter.
Corresponding author
Ethics declarations
Ethics approval
The project was approved by the Research Ethics Committee (CAAE 31264220.0.1001.5264).
Consent to participate
Patients and/or their relatives signed a consent form to participate in the study.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(XLSX 39 kb)
Rights and permissions
About this article
Cite this article
Goudouris, E.S., Pinto-Mariz, F., Mendonça, L.O. et al. Outcome of SARS-CoV-2 Infection in 121 Patients with Inborn Errors of Immunity: A Cross-Sectional Study. J Clin Immunol 41, 1479–1489 (2021). https://doi.org/10.1007/s10875-021-01066-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-021-01066-8