Abstract
Objective
To gain a deeper understanding of the information requirements of clinicians conducting neonatal resuscitation in the first 10 min after birth.
Background
During the resuscitation of a newborn infant in the first minutes after birth, clinicians must monitor crucial physiological adjustments that are relatively unobservable, unpredictable, and highly variable. Clinicians’ access to information regarding the physiological status of the infant is also crucial to determining which interventions are most appropriate. To design displays to support clinicians during newborn resuscitation, we must first carefully consider the information requirements.
Methods
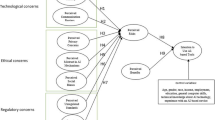
We conducted a work domain analysis (WDA) for the neonatal transition in the first 10 min after birth. We split the work domain into two ‘subdomains’; the physiology of the neonatal transition, and the clinical resources supporting the neonatal transition. A WDA can reveal information requirements that are not yet supported by resources.
Results
The physiological WDA acted as a conceptual tool to model the exact processes and functions that clinicians must monitor and potentially support during the neonatal transition. Importantly, the clinical resources WDA revealed several capabilities and limitations of the physical objects in the work domain—ultimately revealing which physiological functions currently have no existing sensor to provide clinicians with information regarding their status.
Conclusion
We propose two potential approaches to improving the clinician’s information environment: (1) developing new sensors for the information we lack, and (2) employing principles of ecological interface design to present currently available information to the clinician in a more effective way.



Similar content being viewed by others
References
Cheong JL, Doyle LW. Increasing rates of prematurity and epidemiology of late preterm birth. J Paediatr Child Health. 2012;48(9):784–8. https://doi.org/10.1111/j.1440-1754.2012.02536.x.
Wyllie J, Perlman JM, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, Kim HS, Liley HG, Mildenhall L, Simon WM, Szyld E, Tamura M, Velaphi S. International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2015;95:e169–201. https://doi.org/10.1016/j.resuscitation.2015.07.045.
Izquierdo M, Iriondo M, Ruiz C, Zeballos G, Sánchez M, González E, Vento M, Thió M. Survey of neonatal resuscitation practices showed post-training improvements but need to reinforce preterm management, monitoring and adrenaline use. Acta Paediatr. 2017;106(6):897–903. https://doi.org/10.1111/apa.13791.
Charles E, Hunt K, Murthy V, Harris C, Greenough A. UK neonatal resuscitation survey. Arch Dis Child Fetal Neonatal Ed. 2018;70:F1–2. https://doi.org/10.1136/archdischild-2018-315526.
McCarthy LK, Morley CJ, Davis PG, Kamlin COF, O’Donnell CPF. Timing of interventions in the delivery room: does reality compare with neonatal resuscitation guidelines? J Pediatr. 2013;163(6):1553-7.e1551. https://doi.org/10.1016/j.jpeds.2013.06.007.
Mumma JM, Durso FT, Dyes M, Dela Cruz R, Fox VP, Hoey M. Bag valve mask ventilation as a perceptual-cognitive skill. Hum Factors. 2018;60(2):212–21. https://doi.org/10.1177/0018720817744729.
Trevisanuto D, De Bernardo G, Res G, Sordino D, Doglioni N, Weiner G, Cavallin F. Time perception during neonatal resuscitation. J Pediatr. 2016;177:103–7. https://doi.org/10.1016/j.jpeds.2016.07.003.
Yamada NK, Kamlin COF, Halamek LP. Optimal human and system performance during neonatal resuscitation. Semin Fetal Neonatal Med. 2018;23(5):306–11. https://doi.org/10.1016/j.siny.2018.03.006.
Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, Donath SM, Davis PG, Morley CJ. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010;125(6):e1340–7. https://doi.org/10.1542/peds.2009-1510.
Liley HG, Mildenhall L, Morley P. Australian and New Zealand Committee on Resuscitation Neonatal Resuscitation guidelines 2016. J Paediatr Child Health. 2017;53(7):621–7.
Manley BJ, Owen LS, Hooper S, Jacobs SE, Cheong JL, Doyle LW, Davis PG. Towards evidence-based resuscitation of the newborn infant. Lancet. 2017;389(10079):1639–48.
Askin DF. Complications in the transition from fetal to neonatal life. J Obstetr Gynecol Neonatal Nurs. 2002;31(3):318–27.
Hillman NH, Kallapur SG, Jobe AH. Physiology of transition from intrauterine to extrauterine life. Clinics in perinatology. 2012;39(4):769–83.
Hooper S, Kitchen MJ, Polglase GR, Roehr CC, te Pas AB. The physiology of neonatal resuscitation. Curr Opin Pediatr. 2018;30(2):187–91.
Roehr C, Hansmann G, Hoehn T, Buhrer C. The 2010 Guidelines on Neonatal Resuscitation (AHA, ERC, ILCOR): similarities and differences–what progress has been made since 2005. Klin Padiatr. 2011;223(5):299–307.
McLanders ML, Marshall SD, Sanderson PM, Liley HG. The cognitive aids in medicine assessment tool (CMAT) applied to five neonatal resuscitation algorithms. J Perinatol. 2016;37:387. https://doi.org/10.1038/jp.2016.235.
Calder LA, Bhandari A, Mastoras G, Day K, Momtahan K, Falconer M, Weitzman B, Sohmer B, Cwinn AA, Hamstra SJ, Parush A. Healthcare providers’ perceptions of a situational awareness display for emergency department resuscitation: a simulation qualitative study. Int J Qual Health Care. 2018;30(1):16–22. https://doi.org/10.1093/intqhc/mzx159.
Grundgeiger T, Huber S, Reinhardt D, Steinisch A, Happel O, Wurmb T. Cognitive aids in acute care. Paper presented at the CHI Conference on Human Factors in Computing Systems; (2019)
Schild S, Sedlmayr B, Schumacher AK, Sedlmayr M, Prokosch HU, St.pierre M,. A digital cognitive aid for anesthesia to support intraoperative crisis management: Results of the user-centered design process. JMIR mHealth uHealth. 2019. https://doi.org/10.2196/13226.
Gonzales M, Henry J, Calhoun A, Riek L. Visual TASK: A Collaborative Cognitive Aid for Acute Care Resuscitation. arXivorg. (2016)
Yamada NK, Fuerch JH, Halamek LP. (2019) Ergonomic challenges inherent in neonatal resuscitation Children 74 (6):1–11. https://doi.org/10.3390/children6060074.
Vicente KJ, Rasmussen J. Ecological interface design: theoretical foundations. Systems, Man and Cybernetics. IEEE Transactions on. 1992;22(4):589–606. https://doi.org/10.1109/21.156574.
Naikar N. (2016) Work Domain Analysis: Concepts, Guidelines, and Cases. CRC Press Inc - M.U.A.
Jamieson GA. Bridging the gap between cognitive work analysis and ecological interface design. In: Proceedings of the Human Factors and Ergonomics Society Annual Meeting, 2003. vol 3. SAGE Publications Sage CA: Los Angeles, CA, pp 273–277.
Reising DVC, Sanderson PM. Work domain analysis and sensors I: principles and simple example. Int J Hum Comput Stud. 2002;56(6):569–96.
Reising DVC, Sanderson PM. Work domain analysis and sensors II: Pasteurizer II case study. International Journal of Human-computer studies. 2002;56(6):597–637.
Hajdukiewicz JR, Burns CM, Vicente KJ. Eggleston RG Work domain analysis for intentional systems. In: Proceedings of the Human Factors and Ergonomics Society Annual Meeting, 1999. vol 3. SAGE Publications Sage CA: Los Angeles, CA, pp 333–337.
Thompson LK, Hickson JC. Burns CM A work domain analysis for diabetes management. In: Proceedings of the Human Factors and Ergonomics Society Annual Meeting, 2003. vol 12. SAGE Publications Sage CA: Los Angeles, CA, pp 1516–1520.
Watson MO, Sanderson P Work domain analysis for the evaluation of human interaction with anaesthesia alarm systems. In: Computer Human Interaction Conference, 1998. Proceedings. 1998 Australasian, 1998. IEEE, pp 228–235.
Sharp TD, Helmicki AJ. The application of the ecological interface design approach to neonatal intensive care medicine. In: Proceedings of the Human Factors and Ergonomics Society Annual Meeting, 1998. vol 3. SAGE Publications Sage CA: Los Angeles, CA, pp 350–354. https://doi.org/10.1177/154193129804200336.
Burns CM, Hajdukiewicz JR. Ecological interface design. USA: CRC Press; 2004.
Watson MO, Sanderson PM. Designing for attention with sound: Challenges and extensions to ecological interface design. Hum Factors. 2007;49(2):331–46.
Hooper S, te Pas AB, Kitchen MJ. (2015) Respiratory transition in the newborn: a three-phase process. Archives of Disease in Childhood-Fetal and Neonatal Edition:fetalneonatal-2013-305704.
Te Pas AB, Davis PG, Hooper S, Morley CJ. From Liquid to Air: Breathing after Birth. The Journal of Pediatrics. 2008;152(5):607–11. https://doi.org/10.1016/j.jpeds.2007.10.041.
Blackburn ST. Maternal, fetal, & neonatal physiology. Maternal, fetal, and neonatal physiology. Fifth ed. edn. St. Louis: Elsevier Inc.; 2018.
Hooper S, Polglase GR, Roehr CC. Cardiopulmonary changes with aeration of the newborn lung. Paediatr Respir Rev. 2015;16(3):147–50. https://doi.org/10.1016/j.prrv.2015.03.003.
Hooper S, Arjan BTP, Justin L, Jeroen JVV, Charles Christoph R, Martin K, Andrew WG, Euan MW, Graeme RP. (2015) Cardiovascular transition at birth: a physiological sequence. Pediatr Res 77 (5). https://doi.org/10.1038/pr.2015.21.
Bhatt S, Alison B, Wallace E, Crossley K, Gill A, Kluckow M, Te Pas AB, Morley CJ, Polglase G, Hooper SB. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol-London. 2013;591(8):2113–26. https://doi.org/10.1113/jphysiol.2012.250084.
Kashyap AJ, Hodges RJ, Thio M, Rodgers KA, Amberg BJ, McGillick EV, Hooper SB, Crossley KJ, Dekoninck PLJ. Physiologically based cord clamping improves cardiopulmonary haemodynamics in lambs with a diaphragmatic hernia. Arch Dis Childhood. 2019. https://doi.org/10.1136/archdischild-2019-316906.
Lio A, Aurilia C, Zahra V, Moss TJ, LaRosa DA, Hooper SB, Gill AW, Kluckow M, Nitsos I, Vento G, Polglase GR. Ventilation prior to umbilical cord clamping improves cardiovascular stability and oxygenation in preterm lambs after exposure to intrauterine inflammation (Report). Front Pediatr. 2018. https://doi.org/10.3389/fped.2018.00286.
Polglase GR, Blank DA, Barton SK, Miller SL, Stojanovska V, Kluckow M, Gill AW, Larosa D, Te Pas AB, Hooper SB. Physiologically based cord clamping stabalises cardiac output and reduces cerebrovascular injury in asphyxiated near-term lambs. Arch Dis Childhood. 2018;103(6):530. https://doi.org/10.1136/archdischild-2017-313657.
Michel A, Lowe NK. The successful immediate neonatal transition to extrauterine life. Biol Res Nurs. 2017;19(3):287–94.
Ong T, Sobotka KS, Siew ML, Crossley KJ, van Vonderen JJ, Polglase GR, Hooper SB. The cardiovascular response to birth asphyxia is altered by the surrounding environment. Arch Dis Child Fetal Neonatal Ed. 2016;101(6):p.F540. https://doi.org/10.1136/archdischild-2015-309596.
Potter EK, Parker P, Caine A, Lumbers ER. Potentiation of cardiac vagal action by cold. Clin Sci. 1985;68(2):165–9.
Sobotka KS, Morley C, Ong T, Polglase GR, Aridas JD, Miller SL, Schmölzer GM, Klingenberg C, Moss TJ, Jenkin G. Circulatory responses to asphyxia differ if the asphyxia occurs in utero or ex utero in near-term lambs. PLoS ONE. 2014;9(11):e112264.
Anton O, Fernandez R, Rendon-Morales E, Aviles-Espinosa R, Jordan H, Rabe H. Heart rate monitoring in newborn babies: a systematic review. Neonatology. 2017;116(3):199–210.
Johnson PA, Cheung P-Y, Lee T-F, O’Reilly M, Schmölzer GM. Novel technologies for heart rate assessment during neonatal resuscitation at birth—a systematic review. Resuscitation. 2019;143:196–207.
Kevat AC, Bullen DV, Davis PG, Kamlin COF. A systematic review of novel technology for monitoring infant and newborn heart rate. Acta Paediatr. 2017;106(5):710–20.
Verbeek C, Zanten H, Vonderen J, Kitchen M, Hooper S, Pas A. Accuracy of currently available neonatal respiratory function monitors for neonatal resuscitation. Eur J Pediatr. 2016;175(8):1065–70. https://doi.org/10.1007/s00431-016-2739-1.
van Vonderen JJ, van Zanten HA, Schilleman K, Hooper SB, Kitchen MJ, Witlox RS, te Pas AB. Cardiorespiratory monitoring during neonatal resuscitation for direct feedback and audit. Front Pediatr. 2016;4:38.
Hooper S, Fouras A, Siew ML, Wallace MJ, Kitchen MJ, Te Pas AB, Klingenberg C, Lewis RA, Davis PG, Morley CJ. Expired CO2 levels indicate degree of lung aeration at birth. PLoS ONE. 2013;8(8):e70895.
van Os S, Cheung PY, Pichler G, Aziz K, O’reilly M, Schmölzer GM. Exhaled carbon dioxide can be used to guide respiratory support in the delivery room. Acta Paediatr. 2014;103(8):796–806.
Hughes S, Blake B, Woods S, Lehmann C. False-positive results on colorimetric carbon dioxide analysis in neonatal resuscitation: potential for serious patient harm. J Perinatol. 2007;27(12):800.
Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, Guinsburg R, Hazinski MF, Morley C, Richmond S. Part 11: neonatal resuscitation: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2010;122(16_suppl_2):516–38.
Brusa G, Savoia M, Vergine M, Bon A, Copetti R, Cattarossi L. Neonatal lung sonography. J Ultrasound Med. 2015;34(9):1549–54. https://doi.org/10.7863/ultra.15.14.08016.
Liu J. Lung ultrasonography for the diagnosis of neonatal lung disease. J Maternal Fetal Neonatal Med. 2014;27(8):856–61. https://doi.org/10.3109/14767058.2013.844125.
Raimondi F, Yousef N, Migliaro F, Capasso L, De Luca D. Point-of-care lung ultrasound in neonatology: classification into descriptive and functional applications. Pediatr Res. 2018. https://doi.org/10.1038/s41390-018-0114-9.
Copetti R, Cattarossi L, Macagno F, Violino M, Furlan R. Lung ultrasound in respiratory distress syndrome: a useful tool for early diagnosis. Neonatology. 2008;94(1):52–9. https://doi.org/10.1159/000113059.
Corsini I, Parri N, Gozzini E, Coviello C, Leonardi V, Poggi C, Giacalone M, Bianconi T, Tofani L, Raimondi F, Dani C. Lung ultrasound for the differential diagnosis of respiratory distress in neonates. Neonatology. 2018;115(1):77–84. https://doi.org/10.1159/000493001.
Piastra M, Yousef N, Brat R, Manzoni P, Mokhtari M, De Luca D. Lung ultrasound findings in meconium aspiration syndrome. Early Hum Dev. 2014;90:41–3. https://doi.org/10.1016/S0378-3782(14)50011-4.
Raimondi F, Rodriguez Fanjul J, Aversa S, Chirico G, Yousef N, De Luca D, Corsini I, Dani C, Grappone L, Orfeo L, Migliaro F, Vallone G, Capasso L. Lung ultrasound for diagnosing pneumothorax in the critically Ill neonate. J Pediatr. 2016;175:74-8.e71. https://doi.org/10.1016/j.jpeds.2016.04.018.
Raimondi F, Yousef N, Rodriguez Fanjul J, De Luca D, Corsini I, Shankar-Aguilera S, Dani C, Di Guardo V, Lama S, Mosca F, Migliaro F, Sodano A, Vallone G, Capasso L. A multicenter lung ultrasound study on transient tachypnea of the neonate. Neonatology. 2019;115(3):263–8. https://doi.org/10.1159/000495911.
Blank DA, Hooper SB, Binder-Heschl C, Kluckow M, Gill AW, Larosa DA, Inocencio IM, Moxham A, Rodgers K, Zahra VA, Davis PG, Polglase GR. Lung ultrasound accurately detects pneumothorax in a preterm newborn lamb model. J Paediatr Child Health. 2016;52(6):643–8. https://doi.org/10.1111/jpc.13154.
Brat R, Yousef N, Klifa R, Reynaud S, Shankar Aguilera S, De Luca D. Lung ultrasonography score to evaluate oxygenation and surfactant need in neonates treated with continuous positive airway pressure. JAMA Pediatr. 2015;169(8):e151797. https://doi.org/10.1001/jamapediatrics.2015.1797.
Martino LD, Yousef N, Ben-Ammar R, Raimondi F, Shankar-Aguilera S, Luca DD. Lung ultrasound score predicts surfactant need in extremely preterm neonates. (Report). Pediatrics. 2018. https://doi.org/10.1542/peds.2018-0463.
Raimondi F, Migliaro F, Sodano A, Umbaldo A, Romano A, Vallone G, Capasso L. Can neonatal lung ultrasound monitor fluid clearance and predict the need of respiratory support? Crit Care. 2012;16(6):R220. https://doi.org/10.1186/cc11865.
Sim S-S, Lien W-C, Chou H-C, Chong K-M, Liu S-H, Wang C-H, Chen S-Y, Hsu C-Y, Yen Z-S, Chang W-T, Huang C-H, Ma MH-M, Chen S-C. Ultrasonographic lung sliding sign in confirming proper endotracheal intubation during emergency Intubation. Resuscitation. 2012;83(3):307–12. https://doi.org/10.1016/j.resuscitation.2011.11.010.
Blank DA, Kamlin COF, Rogerson SR, Fox LM, Lorenz L, Kane SC, Polglase GR, Hooper SB, Davis PG. Lung ultrasound immediately after birth to describe normal neonatal transition: an observational study. Arch Dis Childhood. 2018;103(2):F157. https://doi.org/10.1136/archdischild-2017-312818.
Miedema M, Frerichs I, de Jongh FHC, van Veenendaal MB, van Kaam AH. Pneumothorax in a preterm infant monitored by electrical impedance tomography: a case report. Neonatology. 2010;99(1):10–3. https://doi.org/10.1159/000292626.
Steinmann D, Engehausen M, Stiller B, Guttmann J. Electrical impedance tomography for verification of correct endotracheal tube placement in paediatric patients: a feasibility study. Acta Anaesthesiol Scand. 2013;57(7):881–7. https://doi.org/10.1111/aas.12143.
van Der Burg PS, Miedema M, de Jongh FH, van Kaam AH. Unilateral atelectasis in a preterm infant monitored with electrical impedance tomography: a case report. Eur J Pediatr. 2014;173(12):1715–7. https://doi.org/10.1007/s00431-014-2399-y.
Johnson K. Ventilation and perfusion scanning in children. Paediatr Respir Rev. 2000;1(4):347–53. https://doi.org/10.1053/prrv.2000.0075.
Sánchez-Crespo EA, Rohdin AM, Carlsson AC, Bergström AS, Larsson AS, Jacobsson AH, Lindahl AS, Jonsson AB. A technique for lung ventilation–perfusion SPECT in neonates and infants. Nucl Med Commun. 2008;29(2):173–7. https://doi.org/10.1097/MNM.0b013e3282f25905.
Frerichs I, Amato MBP, van Kaam AH, Tingay DG, Zhao Z, Grychtol B, Bodenstein M, Gagnon H, Böhm SH, Teschner E, Stenqvist O, Mauri T, Torsani V, Camporota L, Schibler A, Wolf GK, Gommers D, Leonhardt S, Adler A. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax. 2016;72(1):83–93. https://doi.org/10.1136/thoraxjnl-2016-208357.
Frerichs I, Becher T, Weiler N. Electrical impedance tomography imaging of the cardiopulmonary system. Curr Opin Crit Care. 2014. https://doi.org/10.1097/MCC.0000000000000088.
Leonhardt S, Lachmann B. (2012) Electrical impedance tomography: the holy grail of ventilation and perfusion monitoring?, vol 38. https://doi.org/10.1007/s00134-012-2684-z.
Lundin S, Stenqvist O. Electrical impedance tomography: potentials and pitfalls. Curr Opin Crit Care. 2012;18(1):35–41. https://doi.org/10.1097/MCC.0b013e32834eb462.
Miedema M, de Jongh FHC, Frerichs I, van Veenendaal MB, van Kaam AH. Changes in lung volume and ventilation during lung recruitment in high-frequency ventilated preterm infants with respiratory distress syndrome. J Pediatr. 2011;159(2):199-205.e192. https://doi.org/10.1016/j.jpeds.2011.01.066.
Carlisle H, Armstrong R, Davis P, Schibler A, Frerichs I, Tingay D. Regional distribution of blood volume within the preterm infant thorax during synchronised mechanical ventilation. Intens Care Med. 2010;36(12):2101–8. https://doi.org/10.1007/s00134-010-2049-4.
Tingay DG, Wallace MJ, Bhatia R, Schmölzer GM, Zahra VA, Dolan MJ, Hooper SB, Davis PG. Surfactant before the first inflation at birth improves spatial distribution of ventilation and reduces lung injury in preterm lambs. J Appl Physiol (Bethesda Md). 2014;116(3):251–8. https://doi.org/10.1152/japplphysiol.01142.2013.
Alenick DS, Holzman IR, Ritter SB. The neonatal transitional circulation: a combined noninvasive assessment. Echocardiography. 1992;9(1):29–37. https://doi.org/10.1111/j.1540-8175.1992.tb00436.x.
Broadhouse KM, Price AN, Durighel G, Cox DJ, Finnemore AE, Edwards AD, Hajnal JV, Groves AM. Assessment of PDA shunt and systemic blood flow in newborns using cardiac MRI. NMR Biomed. 2013. https://doi.org/10.1002/nbm.2927.
Popat H, Kluckow M. Noninvasive assessment of the early transitional circulation in healthy term infants. Neonatology. 2012;101(3):166–71. https://doi.org/10.1159/000330052.
Randalal M, Eronen M, Andersson S, Pohjavuori M, Pesonen E. Pulmonary artery pressure in term and preterm neonates. Acta Pædiatr. 1996;85(11):1344–7. https://doi.org/10.1111/j.1651-2227.1996.tb13922.x.
Friedberg KM, Su DX, Tworetzky JW, Soriano RB, Powell RA, Marx RG. Validation of 3D echocardiographic assessment of left ventricular volumes, mass, and ejection fraction in neonates and infants with congenital heart disease: a comparison study with cardiac MRI. Circ Cardiovasc Imaging. 2010;3(6):735–42. https://doi.org/10.1161/CIRCIMAGING.109.928663.
Noori S, Drabu B, Soleymani S, Seri I. Continuous non-invasive cardiac output measurements in the neonate by electrical velocimetry: a comparison with echocardiography. Arch Dis Childhood. 2012;97(5):F340. https://doi.org/10.1136/fetalneonatal-2011-301090.
Ficial B, Finnemore AE, Cox DJ, Broadhouse KM, Price AN, Durighel G, Ekitzidou G, Hajnal JV, Edwards AD, Groves AM. Validation study of the accuracy of echocardiographic measurements of systemic blood flow volume in newborn infants. J Am Soc Echocardiogr. 2013;26(12):1365–71. https://doi.org/10.1016/j.echo.2013.08.019.
Goldberg SJ, Allen HD, Sahn DJ. Echocardiographic detection and management of patent ductus arteriosus in neonates with respiratory distress syndrome: A two-and one-half year prospective study. J Clin Ultrasound. 1977;5(3):161–9. https://doi.org/10.1002/jcu.1870050306.
Sahn JD, Allen DH. Real-time cross-sectional echocardiographic imaging and measurement of the patent ductus arteriosus in infants and children. Circulation. 1978;58(2):343–54. https://doi.org/10.1161/01.CIR.58.2.343.
Thankavel PP, Rosenfeld CR, Christie L, Ramaciotti C. Early echocardiographic prediction of ductal closure in neonates 30 weeks gestation. J Perinatol. 2012;33(1):45. https://doi.org/10.1038/jp.2012.41.
van Vonderen JJ, Roest AW, Siew ML, Blom NA, van Lith JM, Walther FJ, Hooper S, te Pas AB. Noninvasive measurements of hemodynamic transition directly after birth. Pediatr Res. 2013;75(3):448. https://doi.org/10.1038/pr.2013.241.
van Vonderen JJ, te Pas AB, Kolster-Bijdevaate C, van Lith JM, Blom NA, Hooper S, Roest AAW. Non-invasive measurements of ductus arteriosus flow directly after birth. Arch Dis Childhood. 2014;99(5):F408. https://doi.org/10.1136/archdischild-2014-306033.
Fuerch JH, Sanderson P, Barshi I, Liley H. Developing safe devices for neonatal care. Semin Perinatol:151176. 2019. https://doi.org/10.1053/j.semperi.2019.08.005.
Janata P, Edwards WH. A novel sonification strategy for auditory display of heart rate and oxygen saturation changes in clinical settings. Hum Factors. 2013;55(2):356–72. https://doi.org/10.1177/0018720812455433.
Sanderson PM, Watson MO, Russell WJ. Advanced patient monitoring displays: tools for continuous informing. Anesth Analg. 2005;101(1):161–8. https://doi.org/10.1213/01.ANE.0000154080.67496.AE. table of contents.
Görges M, Staggers N. Evaluations of physiological monitoring displays: a systematic review. J Clin Monit Comput. 2008;22(1):45.
Jungk A, Thull B, Hoeft A, Rau G. Evaluation of two new ecological interface approaches for the anesthesia workplace. J Clin Monit Comput. 2000;16(4):243–58.
Blike GT, Surgenor SD, Whalen K, Jensen J. Specific elements of a new hemodynamics display improves the performance of anesthesiologists. J Clin Monit Comput. 2000;16(7):485–91.
Albert RW, Agutter JA, Syroid ND, Johnson KB, Loeb RG, Westenskow DR. A simulation-based evaluation of a graphic cardiovascular display. Anesth Analg. 2007;105(5):1303–11.
Zestic J, Brecknell B, Liley H, Sanderson P. A novel auditory display for neonatal resuscitation: laboratory studies simulating pulse oximetry in the first 10 minutes after birth. Hum Factors. 2019;61(1):119–38. https://doi.org/10.1177/0018720818793769.
Gandhi B, Rich W, Finer N. Achieving targeted pulse oximetry values in preterm infants in the delivery room. J Pediatr. 2013;163(2):412–5. https://doi.org/10.1016/j.jpeds.2013.01.010.
Kelley CR. Manual and automatic control: a theory of manual control and its application to manual and to automatic systems. New York: Wiley; 1968.
Jensen R. (1979) Prediction and quickening in perspective flight displays for curved landing approaches. ProQuest Dissertations Publishing.
Sullivan B, Ware C, Plumlee M. Predictive displays for survey vessels. Proc Hum Factors Ergon Soc Annu Meet. 2006;50(22):2424–8. https://doi.org/10.1177/154193120605002216.
Wickens CD, Gempler K, Morphew ME. Workload and reliability of predictor displays in aircraft traffic avoidance. Transport Hum Factors. 2000;2(2):99–126. https://doi.org/10.1207/STHF0202_01.
Siew ML, Kitchen MJ, te Pas AB, Harding R, Hooper SB. (2014) The Lung: Development, Aging and the Environment: Second Edition. https://doi.org/10.1016/B978-0-12-799941-8.00013-4.
Acknowledgements
Support for this research was provided by Jelena Zestic’s research higher degree (RHD) support funds at The University of Queensland, and her Australian Government Research Training Program (RTP) Scholarship. The project was also supported by The University of Queensland School of Psychology Strategic funds. We gratefully acknowledge the contributions of Dr Robert Loeb in the early phases of constructing the work domain analysis, and Dr Callum Gately for verifying aspects of lung ultrasound.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Penelope Sanderson is coinventor of a respiratory sonification display (US patent 7070570, Sanderson and Watson). None of the other authors have conflicts of interest to declare.
Ethical approval
This project has been reviewed by The University of Queensland Office of Research Ethics and is deemed to be exempt from ethics review under the National Statement on Ethical Conduct in Human Research and The University of Queensland Policy (Clearance Number 2018002297). Approval for re-use of the Dawson [9] data underlying Fig. 1 was granted by the Royal Women’s Hospital (Melbourne) Research and Ethics Secretariat.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix

Adapted from Siew et al. [106]
In utero, the fetus exhibits several unique physiological intracardiac and extracardiac shunts. The fetal shunts include: (a) the Ductus Venosus (DV), which shunts most of the umbilical vein flow directly to the inferior vena cava, (b) the Foramen Ovale (FO), which shunts blood from the right atrium to the left atrium, and (c) the Ductus Arteriosus (DA), which shunts blood from the pulmonary artery to the aorta, away from the lungs

Adapted from Siew et al. [106]
A representation of the transition to the neonatal circulatory system—as a consequence of the dramatic fall in pulmonary vascular resistance with the first breaths and increase in pulmonary blood flow. Typically, if the umbilical cord is clamped immediately, the DA shunt becomes bi-directional and then reverses to become a left to right shunt, filling the expanding pulmonary circulation from the aorta, before the functional ductal closure. The cessation of blood flow from the placenta, together with the low portal venous return from the newborn abdominal organs, triggers the functional closure of the DV within minutes of birth, and reduces right atrial pressure. Additionally, the increase in pulmonary blood flow increases pulmonary venous return to the left atrium. Consequently, the left atrial pressure rises, exceeding the falling right atrial pressure and gradually closing the FO

Adapted from Siew et al. [106]
Transition to neonatal circulatory system—cardiovascular transitions following delayed (or physiological) umbilical cord clamping after onset of lung aeration. Recent studies have shown that if umbilical cord clamping is delayed until or after the onset of lung aeration, the right atrium continues to fill from the placental venous return and right ventricular output supports the expanding pulmonary circulation, stabilising pressures in the heart and major vessels and better supporting perfusion of vital systemic organs. Consequently, the fetal shunts remain patent until the umbilical cord is clamped

Adapted from Siew et al. [106]
The neonatal circulatory system once cardiovascular transitions are near complete at about 10 min after birth. The closure of the DA is strongly influenced by increasing oxygen levels and vasoactive mediators, which cause smooth muscle contraction that gradually (over up to 10–15 h) close the lumen of the vessel. Overall, the cardiovascular adaptations serve the purpose of increasing pulmonary blood flow to facilitate an effective match of lung perfusion and ventilation to improve oxygenation, while maintaining perfusion of systemic organs
Rights and permissions
About this article
Cite this article
Zestic, J., Sanderson, P., Dawson, J. et al. Defining information needs in neonatal resuscitation with work domain analysis. J Clin Monit Comput 35, 689–710 (2021). https://doi.org/10.1007/s10877-020-00526-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-020-00526-7