Abstract
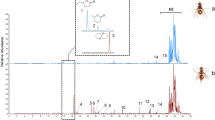
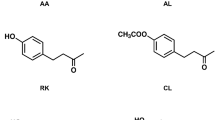
Oriental fruit fly, Bactrocera dorsalis (Hendel), males are highly attracted to the natural phenylpropanoid methyl eugenol (ME). They compulsively feed on ME and metabolize it to ring and side-chain hydroxylated compounds that have both pheromonal and allomonal functions. Side-chain metabolic activation of ME leading to (E)-coniferyl alcohol has long been recognized as a primary reason for hepatocarcinogenicity of this compound in rodents. Earlier, we demonstrated that introduction of a fluorine atom at the terminal carbon of the ME side chain significantly depressed metabolism and specifically reduced formation of coniferyl alcohol but had little effect on field attractiveness to B. dorsalis. In the current paper, we demonstrate that fluorination of ME at the 4 position of the aromatic ring blocks metabolic ring-hydroxylation but overall enhances side-chain metabolism by increasing production of fluorinated (E)-coniferyl alcohol. In laboratory experiments, oriental fruit fly males were attracted to and readily consumed 1,2-dimethoxy-4-fluoro-5-(2-propenyl)benzene (I) at rates similar to ME but metabolized it faster. Flies that consumed the fluorine analog were as healthy post feeding as ones fed on methyl eugenol. In field trials, the fluorine analog I was ∼50% less attractive to male B. dorsalis than ME.





Similar content being viewed by others
Notes
Mention of commercial products does not constitute an endorsement by USDA.
References
Benbow, J. W., and Katoch-Rouse, R. 2001. A biomimethic approach to dihydrobenzofuran synthesis. J. Org. Chem. 66:4965–4972.
Brennan, R. J., Kandikonda, S., Khrimian, A. P., DeMilo, A. B., Liquido, N. J., and Schiestl, R. H. 1996. Saturated and monofluoroanalogs of the oriental fruit fly attractant methyl eugenol show reduced genotoxic activities in yeast. Mutation Res. 369:175–181.
Dean, F. M., Goodchild, J., Houghton, L. E., Martin, J. A., Morton, R. B., Parton, B., Price, A. W., and Somvichien, N. 1966. Boron trichloride as a selective demethylating agent. Tetrahedron Lett. 7:4153–4159.
Furlano, D. C., and Kirk, K. L. 1986. An improved synthesis of 4-fluoroveratrole. Efficient route to 6-fluoroveratraldehyde and 6-fluoro-D,L-DOPA. J. Org. Chem. 51:4073–4075.
Khrimian, A. P., DeMilo, A. B., Waters, R. M., Cunningham, R. T., and Leonhardt, B. A. 1993. Synthesis of attractants for oriental fruit fly Dacus dorsalis Hendel using a catalytic organocopper coupling reaction. J. Chem. Ecol. 19:2935–2946.
Khrimian, A. P., DeMilo, A. B., Waters, R. M., Liquido, N. J., and Nicholson, J. M. 1994. Monofluoroanalogs of eugenol methyl ether as novel attractants for the oriental fruit fly. J. Org. Chem. 59:8034–8039.
Khrimian, A., Jang, E. B., Nagata, J., and Carvalho, L. 2006. Consumption and metabolism of 1,2-dimethoxy-4-(3-fluoro-2-propenyl)benzene, a fluorine analog of methyl eugenol, in the oriental fruit fly, Bactrocera dorsalis (Hendel). J. Chem. Ecol. 32:1513–1526.
Koyama, J., Teruya, T., and Tanaka, K. 1984. Eradication of the oriental fruit fly (Diptera: Tephritidae) from the Okinawa Islands by a male annihilation method. J. Econ. Entomol. 77:468–472.
Liquido, N. J., Khrimian, A. P., DeMilo, A. B., and McQuate, G. T. 1998. Monofluoroanalogs of methyl eugenol: new attractants for males of Bactrocera dorsalis (Hendel) (Dipt., Tephritidae). J. Apll. Entomol. 122:259–264.
Metcalf, R. L. 1990. Chemical ecology of Dacinae fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 83:1017–1030.
Metcalf, R. L., Mitchell, W. C., Fukuto, T. R., and Metcalf, E. R. 1975. Attraction of the oriental fruit fly, Dacus dorsalis, to methyl eugenol and related olfactory stimulants. Proc. Natl. Acad. Sci. USA 72:2501–2505.
Metcalf, R. L., Metcalf, E. R., and Mitchell, W. C. 1981. Molecular parameters and olfaction in the oriental fruit fly Dacus dorsalis. Proc. Natl. Acad. Sci. USA 78:4007–4010.
Miller, E. C., Swanson, A. B., Phillips, D. H., Fletcher, T. L., Liem, A., and Miller, J. A. 1983. Structure-activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 43:1124–1134.
Mitchell, W. C., Metcalf, R. L., Metcalf, E. R., and Mitchell, S. 1985. Candidate substitutes for methyl eugenol as attractants for the area-wide monitoring and control of the oriental fruit fly, Dacus dorsalis Hendel (Diptera: Tephritidae). Environ. Entomol. 14:176–181.
National Toxicology Program 1998. Toxicology and carcinogenesis studies of methyleugenol (CAS No. 93–15–12), in F344/N rats and B6C3F1 mice (gavage studies). Technical Report Series TR-491. US Department of Health and Human Services, Public Health Service, Research Triangle Park.
National Toxicology Program 2002. Report on Carcinogens. 10th edn.US Department of Health and Human Services, Public Health Service, Research Triangle Park.
Nishida, R., Tan, K. H., Serit, M., Lajis, N. H., Sukari, A. M., Takahashi, S., and Fukami, H. 1988. Accumulation of phenylpropanoids in the rectal glands of males of the Oriental fruit fly, Dacus dorsalis. Experentia 44:534–536.
Pan, H. L., Cole, C. A., and Fletcher, T. L. 1980. A facile, rapid preparation of series of cinnamyl alcohols from 3-phenylpropenes using selenium dioxide. Synthesis 1980:813–814.
Quideau, S., and Ralph, J. 1992. Facile large-scale synthesis of coniferyl, sinapyl, and p-coumaryl alcohol. J. Agric. Food Chem. 40:1108–1110.
Sas Institute 1990. SAS/STAT User’s Guide. Release 6.04. SAS Institute, Cary.
Sas Institute 2002. JMP Statistics and Graphics Guide, version 5. SAS Institute Inc., Cary.
Schiestl, R. H., Chan, W. S., Gietz, R. D., Mehta, R. D., and Hastings, P. J. 1989. Safrole, eugenol, and methyl eugenol induce intrachromosomal recombination in yeast. Mutation Res. 224:427–436.
Sekizawa, J., and Shibamoto, T. 1982. Genotoxicity of safrole-related chemicals in microbial test systems. Mutation Res. 101:127–140.
Silverstein, R. M., Bassler, G. C., and Morrill, T. C. 1980. Spectroscopic Identification of Organic Compounds. 4th edn. p. 236. Wiley, New York.
Smith, R. L., Adams, T. B., Doull, J., Feron, V. J., Goodman, J. I., Marnett, L. J., Portoghese, P. S., Waddell, W. J., Wagner, B. M., Rogers, A. E., Caldwell, J., and Sipes, I. G. 2002. Safety assessment of allylalkoxybenzene derivatives used as flavouring substances—methyl eugenol and estragole. Food and Chem. Toxicol. 40:851–870.
Sokal, R. R., and Rohlf, F. J. 1981. Biometry. Freeman, New York.
Statsoft Inc. 2001. STATISTICA (data analysis software system), version 6. www.statsoft.com.
Steiner, L. F. 1952. Methyl eugenol as an attractant for oriental fruit fly. J. Econ. Entomol. 45:241–248.
Steiner, L. F., Mitchel, W. C., Harris, E. J., Kozuma, T. T., and Fujimoto, M. S. 1965. Oriental fruit fly eradication by male annihilation. J. Econ. Entomol. 58:961–964.
Tan, K. H., and Nishida, R. 1996. Sex pheromone and mating competition after methyl eugenol consumption in the Bactrocera dorsalis complex, pp. 147–153, in B. A. McPheron, and G. J. Steck (eds.). Friut Fly Pests. St. Lucie, Delray Beach.
US Department of Agriculture 1983. Host list: oriental fruit fly, Dacus dorsalis. Biological Assessment Support Staff, Plant Protection and Quarantine, USDA, Hyattsville.
Welch, J. T. 1987. Advances in the preparation of biologically active organofluorine compounds. Tetrahedron 43:3123–3197.
Welch, J. T. 1991. The effect of selective fluorination on reactivity in organic and bioorganic chemistry, pp. 1–14, in J. T. Welch (ed.). Selective Fluorination in Organic and Bioorganic ChemistryAmerican Chemical Society, Washington, D.C.
White, I. M., and Elson-Harris, M. M. 1992. Fruit Flies of Economic Significance: Their Identification and Bionomics. CAB International, Wallingford.
Zhang, A., and Amalin, D. 2005. Sex pheromone of the female pink hibiscus mealybug, Maconellicoccus hirsutus (Green) (Homoptera: Pseudococcidae): Biological activity evaluation. Environ. Entomol. 34:264–270.
Acknowledgments
We thank Peter Bianchi, Taina Burke, Sarah Marshall, Rachel Morton, Charmaine Sylva, and Judy Yoshimoto for technical assistance with the field bioassays.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khrimian, A., Siderhurst, M.S., Mcquate, G.T. et al. Ring-Fluorinated Analog of Methyl Eugenol: Attractiveness to and Metabolism in the Oriental Fruit Fly, Bactrocera Dorsalis (Hendel). J Chem Ecol 35, 209–218 (2009). https://doi.org/10.1007/s10886-008-9581-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9581-5