Abstract
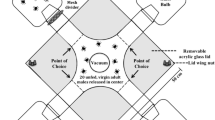
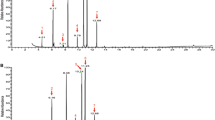
Epicuticular lipids are contact cues in intraspecific chemical communication in insects, both for aggregation and sexual behavior. Triatomine bugs are vectors of the parasite Trypanosoma cruzi, the cause of Chagas disease. In Triatoma infestans, the major epicuticular lipids are hydrocarbons, fatty alcohols, and free and esterified fatty acids. Previously, we found that epicuticular lipid extracts, or selected fatty acid components, trigger aggregation and arrestment behavior in this bug. Using headspace solid phase microextraction, we found no sexual dimorphism in epicuticular hydrocarbons, but found female-specific fatty alcohols (eicosanol and docosanol). The role of epicuticular lipids in T. infestans copulation behavior was tested by observing male responses to live or various treatments of freeze-killed females. We report that hexane-soluble contact cues on females trigger copulation by males. Freeze-killed intact females were attractive to males, but no response was observed when males were exposed to hexane-washed females. Responses were partially recovered when epicuticular extract was applied to the dorsal surface of dead, hexane-washed females. One female equivalent of docosanol, evoked similar responses.



Similar content being viewed by others
References
Arn, H., Rauscher, S., Guerin, P., and Buser, H. R. 1998. Sex pheromone blends of three tortricid pests in European vineyards. Agric. Ecosyst. Environ 21:111–117.
Barbour, J. D., Lacey, E. S., and Hanks, L. M. 2007. Cuticular hydrocarbons mediate mate recognition in a species of longhorned beetle (Coleoptera: Cerambycidae) of the primitive subfamily Prioninae. Ann. Entomol. Soc. Am. 100:333–338.
Bjostad, L. B., Wolf, W. A., and Roelofs, W. L. 1987. Pheromone biosynthesis in lepidopterans: Desaturation and chain-shortening, pp. 77–120, in G. D. Prestwich and G. J. Blomquist (eds.). Pheromone Biochemistry. Academic Press, Orlando, Florida.
Blomquist, G. J., Chu, A. J., and Remaley, S. 1980. Biosynthesis of wax in the honey bee, Apis mellifera L. Insect Biochem. 10:313–321.
Buckner, J. S. 1993. Cuticular polar lipids of insects, pp. 227–270, in D. W. Stanley-Samuelson and D. R. Nelson (eds.). Insect Lipids: Chemistry, Biochemistry and Biology. University of Nebraska Press, Lincoln, Nebraska.
Byrne, D. N. and Hardley, N. F. 1988. Particulate surface waxes of whiteflies: morphology, compositions and waxing behaviour. Physiol. Entomol. 13:267–276.
Calderón-Fernández, G. M., Girotti, J. R., and Juárez M. P. 2011. Cuticular hydrocarbons of Triatoma dimidiata (Hemiptera: Reduviidae): intraspecific variation and chemotaxonomy. J. Med. Entomol. 48: in press.
Crespo, J. G. and Manrique, G. 2007. Mating behavior of the hematophagous bug Triatoma infestans: role of Brindley’s and metaesternal glands. J. insect Physiol. 53:708–714.
Donze, G., Schnyder-Candrian, S., Bogdanov, S., Diehl, P. A., Guerin, P. M., Kilchenmann, V., and Monachon, F. 1998. Aliphatic alcohols and aldehydes of the honey bee cocoon induce arrestment behavior in Varroa jacobsoni (Acari: Mesostigmata), an ectoparasite of Apis mellifera. Arch. Insect Biochem. Physiol. 37:129–145.
Ferveur, J. F., Cobb, M., Boukella, H., and Jallon, J. M. 1996. World-wide variation in Drosophila melanogaster sex pheromone: behavioural effects, genetic bases and potential evolutionary consequences. Genetica 97:73–80.
Ginzel, M. D., Moreira, J. A., Ray, A. M., Millar, J. G., and Hanks, L. M. 2006. (Z)-9-Nonacosene major component of the contact sex pheromone of the beetle, Megacyllene caryae. J. Chem. Ecol. 32:435–451.
Gonzales Audino, P., Alzogaray, R. A., Vassena, C., Masuh, H., Fontan, A., Gatti, P., Martinez, A., Camps, F., Cork, A., and Zerba, E. 2007. Volatile compounds secreted by Brindley’s glands of adult Triatoma infestans: identification and biological activity of previously unidentified compounds. J. Vect. Ecol. 32:75–82.
Juárez, M. P. 1994. Inhibition of cuticular lipid synthesis and its effect on insect survival. Arch. Insect Biochem. Physiol. 25:177–191.
Juárez, M. P. 1995. The effect of sublethal doses of insecticides on Triatoma infestans lipid synthesis. Pest. Biochem. Physiol. 52:81–89.
Juárez, M. P. and Blomquist, G. J. 1993. Cuticular hydrocarbons of Triatoma infestans and T. mazzotti. Comp. Biochem. & Physiol. B 106:667–674.
Juárez, M. P. and Brenner, R. R. 1985. The epicuticular lipids of Triatoma infestans, II. Hydrocarbon dynamics. Comp. Biochem. Physiol. 82:793–803.
Juárez, M. P. and Calderón-Fernández, G. 2007. Cuticular hydrocarbons of triatomines. (Review). Comp. Biochem. Physiol. A 147:711–730.
Juárez, M. P., Blomquist, G. J., and Schofield, C. J. 2001. Hydrocarbons of Rhodnius prolixus, a Chagas disease vector. Comp. Biochem. Physiol. B 129:133–146.
Juárez, M. P., Pedrini, N., Girotti, J. R., Mijailovsky, S. J., and Lorenzo Figueiras, A. N. 2008. Trampa para insectos hematófagos, método de control y método de detección de dichos insectos (Triatoma infestans). Patent pending, Argentina. AR068790 INPI Boletín 568:5.
Lorenzo Figueiras, A. L., Girotti, J. R., Mijailovsky, S. J., and Juárez, M. P. 2009. Epicuticular lipids induce aggregation in Chagas disease vectors. Parasit. Vect. 2:8.
Manrique, G. and Lazzari, C. R. 1994. Sexual behaviour and stridulation during mating in Triatoma infestans (Hemiptera: Reduviidae). Mem. Inst. Oswaldo Cruz 89:629–633.
Pedrini, N., Mijailovsky, S. J., Girotti, J. R., Stariolo, R., Cardozo, R. M., Gentile, A., and Juárez, M. P. 2009. Control of pyrethroid-resistant Chagas disease vectors with entomopathogenic fungi. PLoS Negl. Trop. Dis. 3:e434.
Pickett, J. A., Williams, I. H., and Martin, A. P. 1982. (Z)-11-eicosen-1-ol, an important new pheromonal component from the sting of the honey bee, Apis mellifera L. (Hymenoptera, Apidae.). J. Chem. Ecol. 8:163–175.
Rutledge, C. E., Millar, J. G., Romero, C. M., and Hanks, L. M. 2009. Identification of an Important Component of the Contact Sex Pheromone of Callidiellum rufipenne (Coleoptera: Cerambycidae). Environ. Entomol. 38:1267–1275.
Schal, C., Burns, E. L., Jurenka, R. A., and Blomquist, G. J. 1990. A new component of the female sex pheromone of Blattella germanica (L.) (Dictyoptera: Blattellidae), and interaction with other pheromone components. J. Chem. Ecol. 16:1997–2008.
Schofield, C. J., and Kabayo, J. P. 2008. Trypanosomiasis vector control in Africa and Latin America. Parasit. Vect. 1:24.
Sokal, R. R. and Rohlf, F. J. 2001. Biometry. Freeman, New York.
Witzgall, P., Tasin, M., Buser, H. R., Wegner-KIß, G., Mancebon, V. S. M., Ioratti, C., Bäckman, A. C., Bengtsson, M., Lehmann, L., and Francke, W. 2005. New Pheromone Components of the Grapevine Moth Lobesia botrana. J. Chem. Ecol. 31:2923–2932.
Acknowledgements
We thank Raúl Stariolo for providing insects, and Facundo Bozzolo and Cecilia Fuse for rearing insects. The research was supported in part by grants from the Agencia Nacional para la Promocion de la Ciencia y Tecnología, Argentina (PICT 2004–25479 and PICT 2007–01503) to MPJ. Both MPJ and GMCF are members of the Consejo Nacional de Investigaciones Científicas y Técnicas Researcher’s Career.
Author information
Authors and Affiliations
Corresponding author
Additional information
Luciana M. Cocchiararo-Bastias and Sergio J. Mijailovsky contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cocchiararo-Bastias, L.M., Mijailovsky, S.J., Calderon-Fernández, G.M. et al. Epicuticle Lipids Mediate Mate Recognition in Triatoma infestans . J Chem Ecol 37, 246–252 (2011). https://doi.org/10.1007/s10886-011-9927-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-9927-2