Abstract
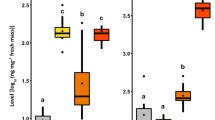
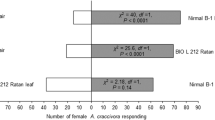
Egg deposition by the Large Cabbage White butterfly Pieris brassicae on Brussels sprouts plants induces indirect defense by changing the leaf surface, which arrests the egg parasitoid Trichogramma brassicae. Previous studies revealed that this indirect defense response is elicited by benzyl cyanide (BC), which is present in the female accessory reproductive gland (ARG) secretion and is released to the leaf during egg deposition. Here, we aimed (1) to elucidate whether P. brassicae eggs induce parasitoid-arresting leaf surface changes in another Brassicacean plant, i.e., Arabidopsis thaliana, and, if so, (2) to chemically characterize the egg-induced leaf surface changes. Egg deposition by P. brassicae on A. thaliana leaves had similar effects to egg deposition on Brussels sprouts with respect to the following: (a) Egg deposition induced leaf surface changes that arrested T. brassicae egg parasitoids. (b) Application of ARG secretion of mated female butterflies or of BC to leaves had the same inductive effects as egg deposition. Based on these results, we conducted GC-MS analysis of leaf surface compounds from egg- or ARG-induced A. thaliana leaves. We found significant quantitative differences in epicuticular waxes compared to control leaves. A discriminant analysis separated surface extracts of egg-laden, ARG-treated, untreated control and Ringer solution-treated control leaves according to their quantitative chemical composition. Quantities of the fatty acid tetratriacontanoic acid (C34) were significantly higher in extracts of leaf surfaces arresting the parasitoids (egg-laden or ARG-treated) than in respective controls. In contrast, the level of tetracosanoic acid (C24) was lower in extracts of egg-laden leaves compared to controls. Our study shows that insect egg deposition on a plant can significantly affect the quantitative leaf epicuticular wax composition. The ecological relevance of this finding is discussed with respect to its impact on the behavior of egg parasitoids.


Similar content being viewed by others
References
Abdel-Latief, M. and Hilker, M. 2008. Innate immunity: eggs of Manduca sexta are able to respond to parasitism by Trichogramma evanescens. Insect Biochem. Mol. Biol. 38:136–145.
Andersson, J., Borg-Karlson, A.-K., and Wiklund, C. 2003. Antiaphrodisiacs in Pierid butterflies: a theme with variation! J. Chem. Ecol. 29:1489–1499.
Avato, P., Bianchi, G., and Pogna, N. 1990. Chemosystematics of surface lipids from maize and some related species. Phytochemistry 29:1571–1576.
Balbyshev, N. F. and Lorenzen, J. H. 1997. Hypersensitivity and egg drop: a novel mechanism of host plant resistance to Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 90:652–657.
Bernays, E. A. and Chapman, R. F. 1997. Host-Plant Selection by Phytophagous Insects. Chapman & Hall, New York.
Beyaert, I., Wäschke, N., Scholz, A., Varama, M., Reinecke, A., and Hilker, M. 2010. Relevance of resource-indicating key volatiles and habitat odour for insect orientation. Anim. Behav. 79:1077–1086.
Bruce, T. J. A., Midega, C. A. O., Birkett, M. A., Pickett, J. A., and Khan, Z. R. 2010. Is quality more important than quantity? Insect behavioural responses to changes in a volatile blend after stemborer oviposition on an African grass. Biol. Lett. 6:314–317.
Chang, G. C., Neufeld, J., Durr, D., Duetting, P. S., and Eigenbrode, S. D. 2004. Waxy bloom in peas influences the performance and behavior of Aphidius ervi, a parasitoid of the pea aphid. Entomol. Exp. Appl. 110:257–265.
Colazza, S., Aquila, G., De Pasquale, C., Peri, E., and Millar, J. G. 2007. The egg parasitoid Trissolcus basalis uses n-nonadecane, a cuticular hydrocarbon from its stink bug host Nezara viridula, to discriminate between female and male hosts. J. Chem. Ecol. 33:1405–1420.
Colazza, S., Lo Bue, M., Lo Giudice, D., and Peri, E. 2009. The response of Trissolcus basalis to footprint contact kairomones from Nezara viridula females is mediated by leaf epicuticular waxes. Naturwissenschaften 96:975–981.
Conti, E., Salerno, G., Leombruni, B., Frati, F., and Bin, F. 2010. Short-range allelochemicals from a plant-herbivore association: a singular case of oviposition-induced synomone for an egg parasitoid. J. Exp. Biol. 213:3911–3919.
Cooper, L. D., Doss, R. P., Price, R., Peterson, K., and Oliver, J. E. 2005. Application of bruchin B to pea pods results in the up-regulation of CYP93C18, a putative isoflavone synthase gene, and an increase in the level of pisatin, an isoflavone phytoalexin. J. Exp. Bot. 56:1229–1237.
Desurmont, G. A. and Weston, P. A. 2011. Aggregative oviposition of a phytophagous beetle overcomes egg-crushing plant defences. Ecol. Entomol. 36:335–343.
Doss, R. P. 2005. Treatment of pea pods with bruchin B results in up-regulation of a gene similar to MtN19. Plant Physiol. Biochem. 43:225–231.
Doss, R. P., Proebsting, W. M., Potter, S. W., and Clement, S. L. 1995. Response of Np mutant of pea (Pisum sativum L.) to pea weevil (Bruchus pisorum L.) oviposition and extracts. J. Chem. Ecol. 21:97–106.
Doss, R. P., Oliver, J. E., Proebsting, W. M., Potter, S. W., Kuy, S. R., Clement, S. L., Williamson, R. T., Carney, J. R., and Devilbiss, E. D. 2000. Bruchins: insect-derived plant regulators that stimulate neoplasm formation. Proc. Natl. Acad. Sci. USA 97:6218–6223.
Dutton, A., Mattiacci, L., Amadò, R., and Dorn, S. 2002. A novel function of the triterpene squalene in a tritrophic system. J. Chem. Ecol. 28:103–116.
Edwards, P. B. 1982. Do waxes on juvenile Eucalyptus leaves provide protection from grazing insects? Aust. J. Ecol. 7:347–352.
Eigenbrode, S. D. 2004. The effects of plant epicuticular waxy blooms on attachment and effectiveness of predatory insects. Arthropod. Struct. Dev 33:91–102.
Eigenbrode, S. D. and Espelie, K. E. 1995. Effects of plant epicuticular lipids on insect herbivores. Annu. Rev. Entomol. 40:171–194.
Espelie, K. E., Bernays, E. A., and Brown, J. J. 1991. Plant and insect cuticular lipids serve as behavioral cues for insects. Arch. Insect Biochem. 17:223–233.
Fatouros, N. E., Bukovinszkine’Kiss, G., Kalkers, L. A., Soler Gamborena, R., Dicke, M., and Hilker, M. 2005. Oviposition-induced plant cues: do they arrest Trichogramma wasps during host location? Entomol. Exp. Appl. 115:207–215.
Fatouros, N. E., Broekgaarden, C., Fatouros, N. E., Broekgaarden, C., Bukovinszkine’Kiss, G., Van Loon, J. J. A., Mumm, R., Huigens, M. E., Dicke, M., and Hilker, M. 2008. Male-derived butterfly anti-aphrodisiac mediates indirect plant defense. Proc. Natl. Acad. Sci. USA 105:10033–10038.
Fatouros, N. E., Pashalidou, F. G., Aponte Cordero, W. V., Van Loon, J. J. A., Mumm, R., Dicke, M., Hilker, M., and Huigens, M. E. 2009. Anti-aphrodisiac compounds of male butterflies increase the risk of egg parasitoid attack by inducing plant synomone production. J. Chem. Ecol. 35:1373–1381.
Feltwell, J. 1982. Large White Butterfly: The Biology, Biochemistry and Physiology of Pieris brassicae (Linnaeus). Dr. W. Junk, The Hague.
Greany, P. D., Tumlinson, J. H., Chambers, D. L., and Boush, G. M. 1977. Chemically mediated host finding by Biosteres (Opius) longicaudatus, a parasitoid of tephritid fruit fly larvae. J. Chem. Ecol. 10:1251–1264.
Hilker, M. and Meiners, T. 2006. Early herbivore alert: insect eggs induce plant defense. J. Chem. Ecol. 32:1379–1397.
Hilker, M. and Meiners, T. 2010. How do plants “notice” attack by herbivorous arthropods? Biol. Rev. 85:267–280.
Hilker, M. and Meiners, T. 2011. Plants and insect eggs: how do they affect each other? Phytochemistry 72:1612–1623.
Jenks, M. A., Tuttle, H. A., Eigenbrode, S. D., and Feldmann, K. A. 1995. Leaf epicuticular waxes of eceriferum mutants in Arabidopsis. Plant Physiol. 108:369–377.
Jenks, M. A., Rashotte, A. M., Tuttle, H. A., and Feldmann, K. A. 1996a. Mutants in Arabidopsis thaliana altered in epicuticular wax and leaf morphology. Plant Physiol. 110:377–385.
Jenks, M. A., Tuttle, H. A., and Feldman, K. A. 1996b. Changes in epicuticular waxes on wild type and eceriferum mutants in Arabidopsis during development. Phytochemistry 42:29–34.
Jenks, M. A., Eigenbrode, S. D., and Lemieux, B. 2002. Cuticular waxes of Arabidopsis, pp. 1–24, in C. Somerville and E. Meyerowitz (eds.), The Arabidopsis Book 1: e0016. American Society of Plant Biologists, Rockville.
Jetter, E. and Schäffer, S. 2001. Chemical composition of the Prunus laurocerasus leaf surface. Dynamic changes of the epicuticular wax film during leaf development. Plant Physiol. 126:1725–1737.
Jetter, R., Schäffer, S., and Riederer, M. 2000. Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant Cell Environ. 23:619–628.
Jetter, R., Kunst, L., and Samuels, A. L. 2006. Composition of plant cuticular waxes, pp. 145–181, in M. Riederer and C. Müller (eds.), Biology of the Plant Cuticle. Annual Plant Reviews, Vol. 23. Blackwell Publishing, Oxford.
Kerstiens, G. 1996. Plant Cuticles: An Integrated Functional Approach. BIOS Scientific Publishers, Oxford.
Knutson, A. 1998. The Trichogramma Manual. b-6071. Texas Agriculture Extension Service Texas A&M University System, College Station.
Koepke, D., Schroeder, R., Fischer, H. M., Gershenzon, J., Hilker, M., and Schmidt, A. 2008. Does egg deposition by herbivorous pine sawflies affect transcription of sesquiterpene synthases in pine? Planta 228:427–438.
Koepke, D., Beyaert, I., Gershenzon, J., Hilker, M., and Schmidt, A. 2010. Species-specific responses of pine sesquiterpene synthases to sawfly oviposition. Phytochemistry 71:909–917.
Kosma, D. K., Bourdenx, B., Bernard, A., Parsons, E. P., Lü, S., Joubès, J., and Jenks, M. A. 2009. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 151:1918–1929.
Kováts, E. 1965. Gas chromatographic characterization of organic substances in the retention index system. Adv. Chromatogr. 1:229–247.
Kunst, L. and Samuels, A. L. 2003. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 42:51–80.
Little, D., Gouhier-Darimont, C., Bruessow, F., and Reymond, P. 2007. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol. 143:784–800.
Lo Giudice, D., Peri, E., Lo Bue, M., and Colazza, S. 2010. Plant surfaces of vegetable crops mediate interactions between chemical footprints of true bugs and their egg parasitoids. Commun. Integr. Biol. 3:70–74.
Müller, C. 2006. Plant-insect interactions on cuticular surfaces, pp. 398–422, in M. Riederer and C. Müller (eds.), Biology of the plant cuticle. Annual plant reviews, vol. 23. Blackwell Publishing, Oxford.
Müller, C. 2008. Resistance at the plant cuticle, pp. 107–129, in A. Schaller (ed.), Induced Plant Resistance to Herbivory. Springer, Berlin.
Noldus, L. P. J. J., van Lenteren, J. C., and Lewis, W. J. 1991. How Trichogramma parasitoids use moth sex pheromones as kairomones: orientation behaviour in a wind tunnel. Physiol. Entomol. 16:313–327.
Petzold-Maxwell, J., Wong, S., Arellano, C., and Gould, F. 2011. Host plant direct defence against eggs of its specialist herbivore, Heliothis subflexa. Ecol. Entomol. 36:700–708.
Pinto, J. D. and Stouthammer, R. 1994. Systematics of the Trichogrammatidae with emphasis on Trichogramma, pp. 1–36, in E. Wajnberg and S. A. Hassan (eds.), Biological Control with Egg Parasitoids. CAB International, Wallingford.
Reifenrath, K., Riederer, M., and Müller, C. 2005. Leaf surface wax layers of Brassicaceae lack feeding stimulants for Phaedon cochleariae. Entomol. Exp. Appl. 115:41–50.
Riederer, M. and Müller, C. 2006. Biology of the Plant Cuticle. Blackwell Publishing, Oxford.
Riederer, M. and Schneider, G. 1990. The effect of the environment on the permeability and composition of Citrus leaf cuticles. Planta 180:154–165.
Rostás, M. and Woelfling, M. 2009. Caterpillar footprints as host location kairomones for Cotesia marginiventris. J. Chem. Ecol. 35:20–27.
Rostás, M., Ruf, D., Zabka, V., and Hildebrandt, U. 2008. Plant surface wax affects parasitoid’s response to host footprints. Naturwissenschaften 95:997–1002.
Rutledge, C. E. 1996. A survey of identified kairomones and synomones used by insect parasitoids to locate and accept their hosts. Chemoecology 7:121–131.
Samuels, L., Kunst, L., and Jetter, R. 2008. Sealing plant surfaces: cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 59:683–707.
Schroeder, R., Wurm, L., Varama, M., Meiners, T., and Hilker, M. 2008. Unusual mechanims involved in learning of oviposition-induced host plant odours in an egg parasitoid? Anim. Behav. 75:1423–1430.
Seino, Y., Suzuki, Y., and Sogawa, K. 1996. An ovicidal substance produced by rice plants in response to oviposition by the whitebacked planthopper, Sogatella furcifera (Horváth) (Homoptera: Delphacidae). Appl. Entomol. Zool. 31:467–473.
Shapiro, A. M. and de Vay, J. E. 1987. Hypersensitivity reaction of Brassica nigra L. (Cruciferae) kills eggs of Pieris butterflies (Lepidoptera: Pieridae). Oecologia 71:631–632.
Shepherd, T. and Griffiths, D. W. 2006. The effect of stress on plant cuticular waxes. New Phytol. 171:469–499.
Shu, S., Swedenborg, P. D., and Jones, R. L. 1990. A kairomone for Trichogramma nubilale (Hymenoptera: Trichogrammatidae). Isolation, identification and synthesis. J. Chem. Ecol. 16:521–529.
Sokal, R. R. and Rohlf, J. F. 1969. Biometry. W. H. Freeman and Co., San Francisco.
Suzuki, Y., Sogawa, K., and Seino, Y. 1996. Ovicidal reaction of rice plants against the Whitebacked planthopper Sogatella furcifera Horváth (Homoptera: Delphacidae. Appl. Entomol. Zool. 31:111–118.
Tamiru, A., Bruce, T. J. A., Woodcock, C. M., Caulfield, J. C., Midega, C. A. O., Ogol, C. K. P. O., Mayon, P., Birkett, M. A., Pickett, J. A., and Khan, Z. R. 2011. Maize landraces recruit egg and larval parasitoids in response to egg deposition by a herbivore. Ecol. Lett. 14:1075–1083.
Wen, M. and Jetter, R. 2009. Composition of secondary alcohols, ketones, alkanediols, and ketols in Arabidopsis thaliana cuticular waxes. J. Exp. Bot. 60:1811–1821.
Acknowledgments
We thank Ute Braun for rearing plants and insects. This work was funded by the German Research Foundation (DFG-GRK 837/2-06) and the Netherlands Organization for Scientific Research NWO/ALW Veni grant 863.09.002 (to N.E.F.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Comparison of cuticular wax composition of leaves of Arabidopsis thaliana in dependence of plant ecotype and extraction method (literature data and own data) (PDF 38 kb)
Rights and permissions
About this article
Cite this article
Blenn, B., Bandoly, M., Küffner, A. et al. Insect Egg Deposition Induces Indirect Defense and Epicuticular Wax Changes in Arabidopsis thaliana . J Chem Ecol 38, 882–892 (2012). https://doi.org/10.1007/s10886-012-0132-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-012-0132-8