Abstract
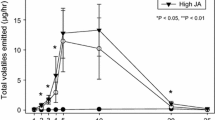
We evaluated the costs and benefits of continuous high-level expression of defenses relative to naturally-induced defenses in field-grown Datura wrightii in the presence and absence of herbivores. We induced D. wrightii plants with monthly applications of the plant hormone methyl jasmonate (MeJA) and assessed levels of inducible proteinase inhibitors (Pins). MeJA application increased Pin production by 124 %, whereas the increase in Pins due to herbivory was more modest (36 %). Pin induction was costly and significantly reduced plant fitness compared to unmanipulated plants both in the presence and absence of herbivores. Although MeJA-treated plants exposed to herbivory suffered significantly less herbivore damage than unmanipulated plants exposed to herbivory, this was not accompanied by a corresponding fitness benefit. In contrast to glasshouse studies in which protected plants never expressed Pins, Pin induction occurred in field-grown plants not treated with MeJA and completely protected from herbivory. Subsequent experiments confirmed that putative herbivore defenses can be induced abiotically in D. wrightii as: 1) Pin levels did not differ significantly between field-grown plants protected from herbivory and plants exposed to chronic herbivory over the full season; and 2) plants exposed to ambient UV-B light in the absence of herbivory expressed low levels of Pins after two wk of exposure, whereas plants protected from UV-B remained uninduced. The costs of induced responses may be relatively easily determined under field conditions, but there may be many inducing agents in the field, and the benefits of induction may be difficult to associate with any single inducing agent.




Similar content being viewed by others
References
Agrawal, A. A. 2000. Benefits and costs of induced plant defense for Lepidium virginicum (Brassicaceae). Ecology 81:1804–1813.
Baldwin, I. T. 1998. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl. Acad. Sci. USA 95:8113–8118.
Barton, K. E. and Koricheva, J. 2010. The ontogeny of plant defense and herbivory: Characterizing general patterns using meta-analysis. Am. Nat. 175:481–493.
Cipollini, D. 2007. Consequences of the overproduction of methyl jasmonate on seed production, tolerance to defoliation and competitive effect and response of Arabidopsis thaliana. New Phytol. 173:146–153.
Cipollini, D. and Heil, M. 2010. Costs and benefits of induced resistance to herbivores and pathogens in plants. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 5:1–25.
Cipollini, D. F. 1997. Wind-induced mechanical stimulation increases pest resistace in common bean. Oecologia 111:84–90.
Conconi, A., Smerdon, M. J., Howe, G. A., and Ryan, C. A. 1996. The octadecanoid signalling pathway in plants mediates a response to ultraviolet radiation. Nature 383:826–829.
Demkura, P. V., Abdala, G., Baldwin, I. T., and Ballare, C. L. 2010. Jasmonate-dependent and -independent pathways mediate specific effects of solar ultraviolet-B radiation on leaf phenolics and antiherbivore defense. Plant Physiol. 152:1084–1095.
Dombrowski, J. E. 2003. Salt stress activation of wound-related genes in tomato plants. Plant Physiol. 132:2098–2107.
Elle, E. and Hare, J. D. 2000. No benefit of glandular trichome production in natural populations of Datura wrightii? Oecologia 123:57–65.
Elle, E., van Dam, N. M., and Hare, J. D. 1999. Cost of glandular trichomes, a "resistance" character in Datura wrightii Regel (Solanaceae). Evolution 53:22–35.
English-Loeb, G., Stout, M. J., and Duffey, S. S. 1997. Drought stress in tomatoes: Changes in plant chemistry and potential nonlinear consequences for insect herbivores. Oikos 79:456–468.
Fornoni, J., Valverde, P. L., and Nunez-Farfan, J. 2003. Quantitative genetics of plant tolerance and resistance against natural enemies of two natural populations of Datura stramonium. Evol. Ecol. Res. 5:1049–1065.
Hare, J. D. 2007. Variation in herbivore and methyl jasmonate-induced volatiles among genetic lines of Datura wrightii. J. Chem. Ecol. 33:2028–2043.
Hare, J. D. 2010. Ontogeny and season constrain the production of herbivore-inducible plant volatiles in the field. J. Chem. Ecol. 36:1363–1374.
Hare, J. D. and Elle, E. 2002. Variable impact of diverse insect herbivores on dimorphic Datura wrightii. Ecology 83:2711–2720.
Hare, J. D. and Smith, J. L. 2005. Competition, herbivory, and reproduction of trichome phenotypes of Datura wrightii. Ecology 86:334–339.
Hare, J. D. and Sun, J. 2011. Production of herbivore-induced plant volatiles is constrained seasonally in the field but predation on herbivores is not. J. Chem. Ecol. 37:430–442.
Hare, J. D. and Walling, L. L. 2006. Constitutive and jasmonate-inducible traits of Datura wrightii. J. Chem. Ecol. 32:29–47.
Heil, M. 2004. Induction of two indirect defences benefits lima bean (Phaseolus lunatus, Fabaceae) in nature. J. Ecol. 92:527–536.
Hermsmeier, D., Schittko, U., and Baldwin, I. T. 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol. 125:683–700.
Huang, Y. M., Xiao, B. Z., and Xiong, L. Z. 2007. Characterization of a stress responsive proteinase inhibitor gene with positive effect in improving drought resistance in rice. Planta 226:73–85.
Izaguirre, M. M., Mazza, C. A., Svatos, A., Baldwin, I. T., and Ballare, C. L. 2007. Solar ultraviolet-B radiation and insect herbivory trigger partially overlapping phenolic responses in Nicotiana attenuata and Nicotiana longiflora. Ann. Bot. 99:103–109.
Jongsma, M. A., Bakker, P. L., and Stiekema, W. J. 1993. Quantitative-determination of serine proteinase-inhibitor activity using a radial diffusion assay. Anal. Biochem. 212:79–84.
Karban, R. 2011. The ecology and evolution of induced resistance against herbivores. Funct. Ecol. 25:339–347.
Karban, R. and Baldwin, I. T. 1997. Induced Responses to Herbivory. University of Chicago Press, Chicago, Illinois.
Karban, R. and Myers, J. H. 1989. Induced plant responses to herbivory. Annu. Rev. Ecol. Syst. 20:331–348.
Kessler, A. and Halitschke, R. 2009. Testing the potential for conflicting selection on floral chemical traits by pollinators and herbivores: Predictions and case study. Funct. Ecol. 23:901–912.
Koricheva, J. 2002. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83:176–190.
Laluk, K. and Mengiste, T. 2011. The Arabidopsis extracellular unusual serine protease inhibitor functions in resistance to necrotrophic fungi and insect herbivory. Plant J. 68:480–494.
Rohwer, C. L. and Erwin, J. E. 2008. Horticultural applications of jasmonates: A review. J. Horticult. Sci. Biotechnol. 83:283–304.
SAS INSTITUTE. 2008. Sas for Windows Version 9.2. SAS Institute. Cary, N. C.
Schluter, U., Benchabane, M., Munger, A., Kiggundu, A., Vorster, J., Goulet, M. C., Cloutier, C., and Michaud, D. 2010. Recombinant protease inhibitors for herbivore pest control: A multitrophic perspective. J. Exp. Bot. 61:4169–4183.
Schmidt, D. D. and Baldwin, I. T. 2006. Transcriptional responses of Solanum nigrum to methyl jasmonate and competition: A glasshouse and field study. Funct. Ecol. 20:500–508.
Srinivasan, T., Kumar, K. R. R., and Kirti, P. B. 2009. Constitutive expression of a trypsin protease inhibitor confers multiple stress tolerance in transgenic tobacco. Plant Cell Physiol. 50:541–553.
van Dam, N. M., Hare, J. D., and Elle, E. 1999. Inheritance and distribution of trichome phenotypes in Datura wrightii. J. Hered. 90:220–227.
van Dam, N. M., Horn, M., Mares, M., and Baldwin, I. T. 2001. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J. Chem. Ecol. 27:547–568.
Walters, D. R. 2009. Are plants in the field already induced? Implications for practical disease control. Crop Protect. 28:459–465.
Zangerl, A. R. and Bazazz, F. A. 1992. Theory and pattern in plant defense allocation, pp. 363–391, in R. S. Fritz and E. L. Simms (eds.), Plant Resistance to Herbivores and Pathogens: Ecology, Evolution and Genetics. University of Chicago Press, Chicago, Illinois.
Acknowledgments
We thank T. Burhans, A. Cossette, J. Lobos, B. Musakwa, L. Nguyen, A. Phan, and T. Spencer for assistance in the field and laboratory and D. Cipollini and M. Heil for comments on previous drafts. This research was funded by the National Science Foundation grant DEB 0414181 to J. D. Hare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Esm 1
(DOCX 44 kb)
Rights and permissions
About this article
Cite this article
Kruidhof, H.M., Allison, J.D. & Hare, J.D. Abiotic Induction Affects the Costs and Benefits of Inducible Herbivore Defenses in Datura wrightii . J Chem Ecol 38, 1215–1224 (2012). https://doi.org/10.1007/s10886-012-0168-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-012-0168-9