Abstract
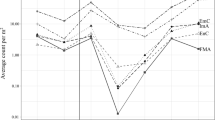
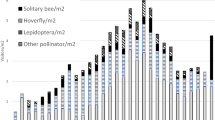
Induction of plant defences can show various levels of localization, which can optimize their efficiency. Locally induced responses may be particularly important in large plants, such as trees, that show high variability in traits and herbivory rates across their canopies. We studied the branch-localized induction of polyphenols, volatiles (VOCs), and changes in leaf protein content in Carpinus betulus L., Quercus robur L., and Tilia cordata L. in a common garden experiment. To induce the trees, we treated ten individuals per species on one branch with methyl jasmonate. Five other individuals per species served as controls. We measured the traits in the treated branches, in control branches on treated trees, and in control trees. Additionally, we ran predation assays and caterpillar food-choice trials to assess the effects of our treatment on other trophic levels. Induced VOCs included mainly mono- and sesquiterpenes. Their production was strongly localized to the treated branches in all three tree species studied. Treated trees showed more predation events than control trees. The polyphenol levels and total protein content showed a limited response to the treatment. Yet, winter moth caterpillars preferred leaves from control branches over leaves from treated branches within C. betulus individuals and leaves from control Q. robur individuals over leaves from treated Q. robur individuals. Our results suggest that there is a significant level of localization in induction of VOCs and probably also in unknown traits with direct effects on herbivores. Such localization allows trees to upregulate defences wherever and whenever they are needed.





Similar content being viewed by others
Data Availability
The data used in this study are accessible at iDiv Data Repository: https://idata.idiv.de/ddm/Data/ShowData/1874. DOI: https://doi.org/10.25829/idiv.1874-3-3124.
References
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11:36–42
Agrawal AA (1999) Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness. Ecology 80:1713–1723
Amo L, Jansen JJ, van Dam NM, Dicke M, Visser ME (2013) Birds exploit herbivore-induced plant volatiles to locate herbivorous prey. Ecol Lett 16:1348–1355
Backmann P, Grimm V, Jetschke G, Lin Y, Vos M, Baldwin IT, van Dam NM (2019) Delayed chemical defense: Timely expulsion of herbivores can reduce competition with neighboring plants. Am Nat 193:125–139
Barbehenn RV, Jaros A, Lee G, Mozola C, Weir Q, Salminen JP (2009) Tree resistance to Lymantria dispar caterpillars: importance and limitations of foliar tannin composition. Oecologia 159:777–788
Barton KE (2016) Tougher and thornier: general patterns in the induction of physical defence traits. Funct Ecol 30:181–187
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bonello P, Blodgett JT (2003) Pinus nigra–Sphaeropsis sapinea as a model pathosystem to investigate local and systemic effects of fungal infection of pines. Physiol Mol Plant Pathol 63:249–261
Clavijo McCormick A et al (2014) Herbivore-induced volatile emission in black poplar: regulation and role in attracting herbivore enemies. Plant Cell Environ 37:1909–1923
Clavijo McCormick A, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310
Danner H, Desurmont GA, Cristescu SM, van Dam NM (2018) Herbivore-induced plant volatiles accurately predict history of coexistence, diet breadth, and feeding mode of herbivores. New Phytol 220:726–738
Defossez E, Pellissier L, Rasmann S (2018) The unfolding of plant growth form-defence syndromes along elevation gradients. Ecol Lett 21:609–618
Dicke M, Loon JJ (2000) Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol Exp Appl 97:237–249
Douma JC, Ganzeveld LN, Unsicker SB, Boeckler GA, Dicke M (2019) What makes a volatile organic compound a reliable indicator of insect herbivory? . Plant Cell Environ 42:3308–3325
Engström MT, Pälijärvi M, Fryganas C, Grabber JH, Mueller-Harvey I, Salminen J-P (2014) Rapid qualitative and quantitative analyses of proanthocyanidin oligomers and polymers by UPLC-MS/MS. J Agric Food Chem 62:3390–3399
Engström MT, Pälijärvi M, Salminen J-P (2015) Rapid fingerprint analysis of plant extracts for ellagitannins, gallic acid, and quinic acid derivatives and quercetin-, kaempferol- and myricetin-based flavonol glycosides by UPLC-QqQ-MS/MS. J Agric Food Chem 63:4068–4079
Eyles A, Bonello P, Ganley R, Mohammed C (2010) Induced resistance to pests and pathogens in trees. New Phytol 185:893–908
Frédérich M, Marcowycz A, Cieckiewicz E, Mégalizzi V, Angenot L, Kiss R (2009) In vitro anticancer potential of tree extracts from the Walloon Region forest. Planta Med 75:1634–1637
Hagerman AE, Butler LG (1978) Protein precipitation method for the quantitative determination of tannins. J Agric Food Chem 26:809–812
Heil M, Ton J (2008) Long-distance signalling in plant defence. Trends Plant Sci 13:264–272
Howe A, Lövei GL, Nachman G (2009) Dummy caterpillars as a simple method to assess predation rates on invertebrates in a tropical agroecosystem. Entomol Exp Appl 131:325–329
Jorge I, Navarro RM, Lenz C, Ariza D, Porras C, Jorrín J (2005) The Holm oak leaf proteome: Analytical and biological variability in the protein expression level assessed by 2-DE and protein identification tandem mass spectrometry de novo sequencing and sequence similarity searching. Proteomics 5:222–234
Kachroo A, Robin GP (2013) Systemic signaling during plant defense. Curr Opin Plant Biol 16:527–533
Kallenbach M, Oh Y, Eilers EJ, Veit D, Baldwin IT, Schuman MC (2014) A robust, simple, high-throughput technique for time‐resolved plant volatile analysis in field experiments. Plant J 78:1060–1072
Kim J, Felton GW (2013) Priming of antiherbivore defensive responses in plants. Insect Sci 20:273–285
Klimm FS, Weinhold A, Volf M (2020) Volatile production differs between oak leaves infested by leaf-miner Phyllonorycter harrisella (Lepidoptera: Gracillariidae) and galler Neuroterus quercusbaccarum (Hymenoptera: Cynipidae). Eur J Entomol 117:101–109
Koricheva J, Nykanen H, Gianoli E (2004) Meta-analysis of trade-offs among plant antiherbivore defenses: Are plants jacks-of-all-trades, masters of all? Am Nat 163:64–75
Kruger NJ (2009) The Bradford method for protein quantitation. In: Walker JM (ed) The protein protocols handbook. Springer Protocols Handbooks. Humana Press, Totowa, pp 17–24
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: Tests in linear mixed effects models. J Stat Softw 82:1–26
Lämke JS, Unsicker SB (2018) Phytochemical variation in treetops: causes and consequences for tree-insect herbivore interactions. Oecologia 187:377–388
Malisch CS, Salminen J-P, Kölliker R, Engström MT, Suter D, Studer B, Lüscher A (2016) Drought effects on proanthocyanidins in sainfoin (Onobrychis viciifolia Scop.) are dependent on the plant’s ontogenetic stage. J Agric Food Chem 64:9307–9316
Mäntylä E, Kleier S, Kipper S, Hilker M (2017) The attraction of insectivorous tit species to herbivore-damaged Scots pines. J Ornithol 158:479–491
Mason CJ, Villari C, Keefover-Ring K, Jagemann S, Zhu J, Bonello P, Raffa KF (2017) Spatial and temporal components of induced plant responses in the context of herbivore life history and impact on host. Funct Ecol 31:2034–2050
Mrazova A, Sam K (2018) Application of methyl jasmonate to grey willow (Salix cinerea) attracts insectivorous birds in nature. Arthropod-Plant Interactions 12:1–8
Murakami M, Yoshida K, Hara H, Toda MJ (2005) Spatio-temporal variation in Lepidopteran larval assemblages associated with oak, Quercus crispula: the importance of leaf quality. Ecol Entomol 30:521–531
Neuvonen S, Haukioja E, Molarius A (1987) Delayed inducible resistance against a leaf-chewing insect in four deciduous tree species. Oecologia 74:363–369
Ohse B (2018) Mutual influences of tree saplings and mammalian herbivores in temperate mixed deciduous forest a functional biodiversity approach. PhD thesis, Universität Leipzig
Piggott N, Ekramoddoullah AK, Liu J-J, Yu X (2004) Gene cloning of a thaumatin-like (PR-5) protein of western white pine (Pinus monticola D. Don) and expression studies of members of the PR-5 group. Physiol Mol Plant Pathol 64:1–8
Posa MRC, Sodhi NS, Koh LP (2007) Predation on artificial nests and caterpillar models across a disturbance gradient in Subic Bay, Philippines. J Trop Ecol 23:27–33
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org, Vienna, Austria
Rasmann S, Bauerle TL, Poveda K, Vannette R (2011) Predicting root defence against herbivores during succession. Funct Ecol 25:368–379
Roden DB, Mattson WJ (2008) Rapid induced resistance and host species effects on gypsy moth, Lymantria dispar (L.): Implications for outbreaks on three tree species in the boreal forest. For Ecol Manag 255:1868–1873
Rostás M, Eggert K (2008) Ontogenetic and spatio-temporal patterns of induced volatiles in Glycine max in the light of the optimal defence hypothesis. Chemoecology 18:29–38
Rubert-Nason KF, Couture JJ, Major IT, Constabel CP, Lindroth RL (2015) Influence of genotype, environment, and gypsy moth herbivory on local and systemic chemical defenses in trembling aspen (Populus tremuloides). J Chem Ecol 41:651–661
Ruuhola T, Yang S, Ossipov V, Haukioja E (2008) Foliar oxidases as mediators of the rapidly induced resistance of mountain birch against Epirrita autumnata Oecologia 154:725–730
Salazar D, Jaramillo A, Marquis RJ (2016) The impact of plant chemical diversity on plant–herbivore interactions at the community level. Oecologia 181:1199–1208
Salminen J-P (2018) Two-dimensional tannin fingerprints by liquid chromatography tandem mass spectrometry offer a new dimension to plant tannin analyses and help to visualize the tannin diversity in plants. J Agric Food Chem 66:9162–9171
Salminen JP, Karonen M (2011) Chemical ecology of tannins and other phenolics: we need a change in approach. Funct Ecol 25:325–338
Salminen JP, Roslin T, Karonen M, Sinkkonen J, Pihlaja K, Pulkkinen P (2004) Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. J Chem Ecol 30:1693–1711
Schultz JC, Baldwin IT (1982) Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217:149–151
Seifert CL, Schulze CH, Dreschke TC, Frötscher H, Fiedler K (2016) Day vs. night predation on artificial caterpillars in primary rainforest habitats–an experimental approach. Entomol Exp Appl 158:54–59
ter Braak CJ, Smilauer P (2012) Canoco reference manual and user’s guide: software for ordination (version 5.0). Microcomputer Power, Ithaca
Travis JMJ, Palmer SCF (2005) Spatial processes can determine the relationship between prey encounter rate and prey density. Biol Let 1:136–138
Tuomi J, Niemela P, Rousi M, Siren S, Vuorisalo T (1988) Induced accumulation of foliage phenols in mountain birch: branch response to defoliation? Am Nat 132:602–608
Turlings TC, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63:433–452
Unsicker SB, Gershenzon J, Köllner TG (2015) Beetle feeding induces a different volatile emission pattern from black poplar foliage than caterpillar herbivory. Plant Signal Behav 10:e987522
Utsumi S, Ohgushi T (2008) Host plant variation in plant-mediated indirect effects: moth boring‐induced susceptibility of willows to a specialist leaf beetle. Ecol Entomol 33:250–260
van Dam NM, Witjes L, Svatoš A (2004) Interactions between aboveground and belowground induction of glucosinolates in two wild Brassica species. New Phytol 161:801–810
Vet LE, Wäckers FL, Dicke M (1991) How to hunt for hiding hosts: the reliability-detectability problem in foraging parasitoids. Neth J Zool 41:202–213
Viswanathan D, Thaler J (2004) Plant vascular architecture and within-plant spatial patterns in resource quality following herbivory. J Chem Ecol 30:531–543
Volf M et al (2018) Community structure of insect herbivores is driven by conservatism, escalation and divergence of defensive traits in Ficus. Ecol Lett 21:83–92
Volf M et al (2019) Quantitative assessment of plant-arthropod interactions in forest canopies: A plot-based approach. PLoS One 14:e0222119
Volf M, Salminen J-P, Segar ST (2019) Evolution of defences in large tropical plant genera: perspectives for exploring insect diversity in a tri-trophic context. Curr Opin Insect Sci 32:91–97
Volf M, Wirth C, van Dam NM (2020) Localized defense induction in trees: a mosaic of leaf traits promoting variation in plant traits, predation, and communities of canopy arthropods? Am J Bot 107:1–4
Acknowledgements
We acknowledge EcoMetEoR – Ecometabolomics Platform for Ecology & Biodiversity Research that supported the VOC analysis. We thank Daniel Uhlig for his help with treating the trees with insecticides and their general maintenance and MIE group members for their support with the data collection. MV acknowledges funding by Alexander von Humboldt Foundation and the Federal Ministry for Education and Research Ref.3.3-CZE-1192673-HFST-P and Grant Agency of the Czech Republic 20-10543Y. AW, HU, CW and NMvD. thank the German Research Foundation for funding the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig (DFG– FZT 118, 202548816). We thank Bettina Ohse, Leuphana University, Germany, for setting up the experimental plot used in this study and Data & Code Unit (iBID) for help with uploading our data to iDiv Data Repository. Finally, we thank Jing Leong for language corrections and helpful comments on the manuscript.
Funding
Alexander von Humboldt Foundation and the Federal Ministry for Education and Research (Ref.3.3-CZE-1192673-HFST-P), Grant Agency of the Czech Republic (20-10543Y), German Research Foundation for funding the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig (DFG– FZT 118, 202548816).
Author information
Authors and Affiliations
Contributions
MV, AW, RR, CW, and NMvD designed the experimental approach; MV, CLS, and TH collected the data; MV, AW, HU, EA, J-P S conducted the chemical analysis, MV conducted the statistical analysis and wrote the first draft of the manuscript; all authors critically contributed to the final draft.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
We declare no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Rights and permissions
About this article
Cite this article
Volf, M., Weinhold, A., Seifert, C.L. et al. Branch-Localized Induction Promotes Efficacy of Volatile Defences and Herbivore Predation in Trees. J Chem Ecol 47, 99–111 (2021). https://doi.org/10.1007/s10886-020-01232-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-020-01232-z