Abstract
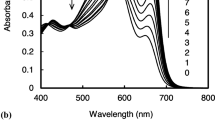
Commercially available CdSe–ZnS Quantum Dots (QDs) have been modified by exchanging the hydrophobic surface ligands with (2-mercaptoethyl)-trimethylammonium chloride. The resulting water soluble conjugate was titrated with solutions of adenosine triphosphate (ATP), adenosine diphosphate, adenosine monophosphate, guanosine triphosphate (GTP), guanosine diphosphate and guanosine monophosphate in 0.01 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer (pH 7.4). A strong fluorescence quench of about 80% was observed for ATP, a quench of 25% was observed for GTP while the others had virtually no effect. The quenching effect of ATP and GTP was attributed to the high negative charge density associated with these substrate’s resulting in a strong attraction to the QD surface enabling them to engage in electron transfer with the excited QD. The lack of fluorescence quenching associated with the other nucleotides was most likely due to their reduced charge density resulting in a lower affinity for the QD surface.





Similar content being viewed by others
References
Parak WJ, Pellegrino T, Plank C (2005) Labeling of cells with quantum dots. Nanotechnology 16:R9
Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Wu AM, Gambir SS, Weiss S (2005) Quantum dots for live cells, in vivo imaging, and diagnostics. Sci 307:538
Giepmans BNG, Adams SR, Ellisman MH, Tsein RY (2006) The fluorescent toolbox for assessing protein location and function. Science 312:217
Callan JF, de Silva AP, Mulrooney RC, McCaughan B (2007) Luminescent sensing with quantum dots. J Incl Phenom Macrocyclic Chem 58:527
Callan JF, Mulrooney RC, Kamila S, McCaughan B (2008) Anion sensing with luminescent quantum dots—a modular approach based on the photoinduced electron transfer (PET) mechanism. J Fluoresc 18:527–532 DOI 10.1007/s10895-007-0295-9
Martinez-Manez R, Sancenon F (2003) Fluorogenic and chromogenic chemosensors and reagents for anions. Chem Rev 103:4419
Burrows CJ, Muller JG (1998) Oxidative nucleobase modifications leading to strand scission. Chem Rev 98:1109
Raymo FM, Yilidiz I (2007) Luminescent chemosensors based on semiconductor quantum dots. Phys Chem Chem Phys 9:2036
Balzani V, Venturi M, Credi A (2003) Molecular devices and machines. Wiley-VCH, Weinheim
Kanekiyo Y, Naganawa R, Yao H (2004) Fluorescence detection of ATP based on the ATP-mediated aggregation of pyrene-appended boronic acid on a polycation. Chem Commun 2004:1006
Schneider SE, O’Neil SN, Anslyn EV (2000) Coupling rational design with libraries leads to the production of an ATP selective chemosensor. J Am Chem Soc 122:542
Ojida A, Park S, Mito-oka Y, Hamachi I (2002) Efficient fluorescent ATP-sensing based on coordination chemistry under aqueous neutral conditions. Tetrahedron Lett 43:6193
Yu WW, Qu L, Guo W, Peng X (2003) Experimental determination of the extinction coefficient of CdTe, CdSe and CdS nanocrystals. Chem Mater 15:2854
de Silva AP, Gunaratne HQN (1990) Fluorescent PET (photoinduced electron transfer) sensors selective for submicromolar calcium with quantitatively predictable spectral and ion-binding properties. J Chem Soc Chem Commun 186:14
de Silva AP, Gunaratne HQN, Lynch PLM (1995) Luminescence and charge transfer.4. On–off fluorescent PET (photoinduced electron transfer) sensors with pyridine receptors-1,3-diaryl- 5-pyridyl-4,5-dihyropyrazoles. J Chem Soc Perkin Trans 2:685
Palaniappan K, Hackney SA, Liu J (2004) Supramolecular control of complexation-induced fluorescence change of water-soluble, beta-cyclodextrin-modified CdS quantum dots. Chem Commun 2004:2704
Acknowledgements
The authors acknowledge financial support from the Robert Gordon University and the Leverhulme Trust UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Callan, J.F., Mulrooney, R.C. & Kamila, S. Luminescent Detection of ATP in Aqueous Solution Using Positively Charged CdSe–ZnS Quantum Dots. J Fluoresc 18, 1157–1161 (2008). https://doi.org/10.1007/s10895-008-0367-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-008-0367-5