Abstract
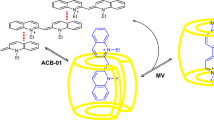
Dye aggregation is detrimental to the performance of high optical density dye-doped photonic materials. To overcome this challenge, the ability of cucurbit[7]uril (CB7) as a molecular host to disrupt aggregate formation on glass substrates was examined. Rhodamine B was covalently attached to glass slides by initially coating the surface with azidohexylsiloxane followed by copper-catalyzed “click” triazole formation with rhodamine B propargyl ester. The absorption and emission spectra of rhodamine B coated slides in water indicated diverse heterogeneous properties as surface dye density varied. Fluorescence quenching due to dye aggregation was evident at high surface dye density. Addition of aqueous cucurbit[7]uril (CB7) to the surface-tethered dyes perturbed the spectra to reveal a considerable reduction in heterogeneity, which suggested that the presence of a surface in close proximity does not significantly impair CB7’s ability to complex with tethered rhodamine B.

Aggregates from densely packed surface-attached rhodamine B exhibited inhomogeneous fluorescence that varies with surface rhodamine B density. CB7 binding disrupted aggregate formation, which led to homogeneous fluorescence independent of the surface density of rhodamine B







Similar content being viewed by others
References
Ooyama Y, Harima Y (2009) Eur J Org Chem 2009:2903
Gratzel M (2005) Inorg Chem 44:6841
de Schryver FC, Vosch T, Cotlet M, van der Auweraer M, Müllen K, Hofkens J (2005) Acc Chem Res 38:514
Tietz C, Jelezko F, Gerken U, Schuler S, Schubert A, Rogl H, Wrachtrup J (2001) Biophys J 81:556
Zhang Y-H, Gao Z-X, Zhong C-L, Zhou H-B, Chen L, Wu W-M, Peng X-J, Yao Z-J (2007) Tetrahedron 63:6813
Kálai T, Hideg K (2006) Tetrahedron 62:10352
Chang PV, Prescher JA, Hangauer MJ, Bertozzi CR (2007) J Am Chem Soc 129:8400
Nau WM, Mohanty J (2005) Int J Photoenergy 7:717
Meier JL, Mercer AC, Rivera H, Burkart MD (2006) J Am Chem Soc 128:12174
Selwyn JE, Steinfeld JI (1972) J Phys Chem 76:762
Rohatgi KK, Singhal GS (1966) J Phys Chem 70:1695
Martyn TA, Moore JL, Halterman RL, Yip WT (2007) J Am Chem Soc 129:10338
Gilliland JW, Yokoyama K, Yip WT (2005) Chem Mater 17:6702
Chambers RW, Kajiwara T, Kearns DR (1974) J Phys Chem 78:380
Abad S, Kluciar M, Miranda MA, Pischel U (2005) J Org Chem 70:10565
Guo X, Zhang D, Zhou Y, Zhu D (2003) J Org Chem 68:5681
Mori T, Ko YH, Kim K, Inoue Y (2006) J Org Chem 71:3232
Bujdak J, Iyi N (2006) J Phys Chem B 110:2180
Gutierrez MC, Hortiguela MJ, Ferrer ML, del Monte F (2007) Langmuir 23:2175
Avnir D, Levy D, Reisfeld R (1984) J Phys Chem 88:5956
Ilich P, Mishra PK, Macura S, Burghardt TP (1996) Spectrochim Acta A 52:1323
Gal ME, Kelly GR, Kurucsev T (1973) J Chem Soc, Faraday Trans 2(69):395
Halterman RL, Moore JL, Mannel LM (2008) J Org Chem 73:3266
Day A, Arnold AP, Blanch RJ, Snushall B (2001) J Org Chem 66:8094
Kim J, Jung I, Kim S, Lee E, Kang J, Sakamoto S, Yamaguchi K, Kim K (2000) J Am Chem Soc 122:540
Lagona J, Mukhopadhyay P, Chakrabarti S, Isaacs L (2005) Angew Chem Int Ed 44:4844
Fiorilli S, Onida B, Barolo C, Viscardi G, Brunel D, Garrone E (2007) Langmuir 23:2261
See for instance, Invitrogen’s “Molecular Probes The Handbook--A guide to fluorescent probes and labeling technologies” http://www.invitrogen.com/site/us/en/home/References/Molecular-Probes-The-Handbook.html
Wang R, Yuan L, Macartney DH (2005) Chem Commun 41:5867
Ong W, Gomez-Kaifer M, Kaifer AE (2002) Org Lett 4:1791
Yuan L, Wang R, Macartney DH (2007) J Org Chem 72:4539
Moon K, Kaifer AE (2004) Org Lett 6:185
Collman JP, Devaraj NK, Chidsey CED (2004) Langmuir 20:1051
Pichon BP, Wong Chi Man M, Bied C, Moreau JJE (2006) J Organomet Chem 691:1126
Hassner A, Alexanian V (1978) Tetrahedron Lett 46:4475
MacBeath G, Koehler AN, Schreiber SL (1999) J Am Chem Soc 121:7967
Gao L, Liu S (2004) Anal Chem 76:7179
Lenhart JL, van Zanten JH, Dunkers JP, Zimba CG, James CA, Pollack SK, Parnas RS (2000) J Colloid Interf Sci 221:75
Bock VD, Hiemstra H, van Maarseveen JH (2006) Eur J Org Chem 2006:51
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) Angew Chem Int Ed 41:2596
DÌez-Gonzalez S, Correa A, Cavallo L, Nolan SP (2006) Chem Eur J 12:7558
del Monte F, Levy D (1998) J Phys Chem B 102:8036
Lee M, Kim J, Tang J, Hochstrasser RM (2002) Chem Phys Lett 359:412
Acknowledgment
The support of NSF (DMR-0805233 to RLH, CHE-0442151 to WTY) is acknowledged as well as a DoEd GAANN fellowship to JLM.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Halterman, R.L., Moore, J.L. & Yip, W.T. Cucurbit[7]uril Disrupts Aggregate Formation Between Rhodamine B Dyes Covalently Attached to Glass Substrates. J Fluoresc 21, 1467–1478 (2011). https://doi.org/10.1007/s10895-011-0832-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-011-0832-4