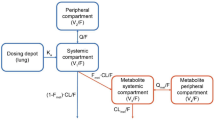
Abstract
The long-acting muscarinic antagonist umeclidinium (UMEC) is approved as a once-daily monotherapy and in combination with the long-acting β2 agonist vilanterol (VI) for chronic obstructive pulmonary disease. The objective of this analysis was to assess the relationship between observed plasma UMEC and/or VI concentrations and QT interval corrected using Fridericia’s correction (QTcF). 103 subjects were enrolled and 86 (83 %) completed the study. Subjects were randomized to 4 of 5 repeat-dose treatments (days 1–10: n = 77 subjects received placebo, n = 76 UMEC 500 µg, n = 78 UMEC/VI 125/25 µg, or n = 76 UMEC/VI 500/100 µg; day 10: n = 74 oral tablet moxifloxacin 400 mg [positive control]). The concentration-QTcF interval relationship was examined using nonlinear mixed-effects methods. For UMEC, predicted QTcF interval prolongation (at observed geometric mean of maximum plasma concentrations) was −2.38 ms (90 % prediction interval [PI] −3.82, −0.85) with UMEC 500 µg and −0.50 ms (90 % PI −0.80, −0.18) and −2.01 ms (90 % PI −3.22, −0.72) with UMEC/VI 125/25 µg and 500/100 µg, respectively. For VI, estimates were 5.89 ms (90 % PI 4.89, 6.91) and 7.23 ms (90 % PI 5.88, 8.55) with UMEC/VI 125/25 µg and 500/100 µg, respectively. Combined additive mean effects were estimated for UMEC/VI 125/25 µg (5.39 ms [90 % PI 4.40, 6.47]) and 500/100 µg (5.22 ms [90 % PI 3.72, 6.80]). The model-predicted decrease with UMEC and increase with UMEC/VI combination in QTcF interval suggest that the QT effect is likely attributable to VI. These model-predicted results support those of previously-published traditional statistical analyses.





Similar content being viewed by others
References
Global Initiative for Chronic Obstructive Lung Disease (2015) Global strategy for the diagnosis, management, and prevention of COPD. www.goldcopd.org. Accessed 9 March 2015
Cazzola M, Centanni S, Santus P, Verga M, Mondoni M, di Marco F, Matera MG (2004) The functional impact of adding salmeterol and tiotropium in patients with stable COPD. Respir Med 98:1214–1221
Cazzola M, Noschese P, Salzillo A, De Giglio C, D’Amato G, Matera MG (2005) Bronchodilator response to formoterol after regular tiotropium or to tiotropium after regular formoterol in COPD patients. Respir Med 99:524–528
van Noord JA, Aumann JL, Janssens E, Smeets JJ, Verhaert J, Disse B, Mueller A, Cornelissen PJ (2005) Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J 26:214–222
van Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, Cornelissen PJ (2006) Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest 129:509–517
Tashkin DP, Littner M, Andrews CP, Tomlinson L, Rinehart M, Denis-Mize K (2008) Concomitant treatment with nebulized formoterol and tiotropium in subjects with COPD: a placebo-controlled trial. Respir Med 102:479–487
Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A (2013) Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med 107:1538–1546
Trivedi R, Richard N, Mehta R, Church A (2014) Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J 43:72–81
De Ponti F, Poluzzi E, Montanaro N (2000) QT-interval prolongation by non-cardiac drugs: lessons to be learned from recent experience. Eur J Clin Pharmacol 56:1–18
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (2005) E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. International Conference on Harmonization. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073153.pdf. Accessed 7 March 2015
Kelleher D, Tombs L, Preece A, Brealey N, Mehta R (2014) A randomized, placebo- and moxifloxacin-controlled thorough QT study of umeclidinium monotherapy and umeclidinium/vilanterol combination in healthy subjects. Pulm Pharmacol Ther 29:49–57
Kelleher D, Tombs L, Crater G, Preece A, Brealey N, Mehta R (2013) A placebo- and moxifloxacin-controlled thorough QT study of umeclidinium monotherapy and umeclidinium/vilanterol combination in healthy subjects. Am J Respir Crit Care Med 187:A1487
Mehta R, Green M, Patel B, Wagg J (2014) Concentration-QT analysis of the randomized, placebo- and moxifloxacin-controlled thorough QT study of inhaled umeclidinium monotherapy and inhaled umeclidinium/vilanterol combination in healthy subjects. Am J Respir Crit Care Med 189:A5981
World Medical Association (2008) WMA Declaration of Helsinki—Ethical principles for medical research involving human subjects. Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and amended (latest) by the 64th WMA General Assembly, Fortaleza, Brazil, October 2013. http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed 7 March 2015
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (1996) International Conference on Harmonisation Tripartite guideline: guidance for good clinical practice E6 (R1). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 7 March 2015
Malik M, Hnatkova K, Schmidt A, Smetana P (2009) Electrocardiographic QTc changes due to moxifloxacin infusion. J Clin Pharmacol 49:674–683
US Food and Drug Administration (1999) Guidance for industry: population pharmacokinetics. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072137.pdf. Accessed 7 March 2015
Committee for Medicinal Products for Human Use (2007) Guideline on reporting the results of population pharmacokinetic analyses. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003067.pdf. Accessed 7 March 2015
Darpo B (2010) The thorough QT/QTc study 4 years after the implementation of the ICH E14 guidance. Br J Pharmacol 159:49–57
Khindri S, Sabo R, Harris S, Woessner R, Jennings S, Drollmann AF (2011) Cardiac safety of indacaterol in healthy subjects: a randomized, multidose, placebo- and positive-controlled, parallel-group thorough QT study. BMC Pulm Med 11:31
Garnett CE, Beasley N, Bhattaram VA, Jadhav PR, Madabushi R, Stockbridge N, Tornøe CW, Wang Y, Zhu H, Gobburu JV (2008) Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48:13–18
Hutmacher MM, Chapel S, Agin MA, Fleishaker JC, Lalonde RL (2008) Performance characteristics for some typical QT study designs under the ICH E-14 guidance. J Clin Pharmacol 48:215–224
Rohatagi S, Carrothers TJ, Kuwabara-Wagg J, Khariton T (2009) Is a thorough QTc study necessary? The role of modeling and simulation in evaluating the QTc prolongation potential of drugs. J Clin Pharmacol 49:1284–1296
Russell T, Riley SP, Cook JA, Lalonde RL (2008) A perspective on the use of concentration-QT modeling in drug development. J Clin Pharmacol 48:9–12
Garg A, Li J, Clark E, Knott A, Carrothers TJ, Marier JF, Cortés J, Brewster M, Visich J, Lum B (2013) Exposure-response analysis of pertuzumab in HER2-positive metastatic breast cancer: absence of effect on QTc prolongation and other ECG parameters. Cancer Chemother Pharmacol 72:1133–1141
Darpo B, Karnad DR, Badilini F, Florian J, Garnett CE, Kothari S, Panicker GK, Sarapa N (2014) Are women more susceptible than men to drug-induced QT prolongation? Concentration-QTc modeling in a Phase 1 study with oral rac-sotalol. Br J Clin Pharmacol 77:522–531
Florian JA, Tornøe CW, Brundage R, Parekh A, Garnett CE (2011) Population pharmacokinetic and concentration—QTc models for moxifloxacin: pooled analysis of 20 thorough QT studies. J Clin Pharmacol 51:1152–1162
Bonate PL (2013) Effect of assay measurement error on parameter estimation in concentration-QTc interval modeling. Pharm Stat 12:156–164
Boehringer Ingelheim Pharmaceuticals, Inc (2014) Spiriva Handihaler prescribing information. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Spiriva/Spiriva.pdf. Accessed 7 March 2015
GSK (2014) Serevent Diskus prescribing information http://us.gsk.com/products/assets/us_serevent_diskus.pdf. Accessed 7 March 2015
Merck Sharpe & Dohme Corp. (2012) Foradil Aerolizer prescribing information. http://www.merck.com/product/usa/pi_circulars/f/foradil/foradil_pi.pdf. Accessed 7 March 2015
Novartis Pharmaceuticals Corporation (2012) Arcapta Neohaler prescribing information http://www.arcapta.com/index.jsp?usertrack.filter_applied=true&NovaId=2935376964246533292. Accessed 7 March 2015
CredibleMeds (2015) Drug lists by risk groups. Drugs that prolong the QT interval and/or induce Torsades de Pointes. http://www.azcert.org/. Accessed 7 March 2015
Kempsford R, Allen A, Kelly K, Saggu P, Crim C (2014) A repeat-dose thorough QT study of inhaled fluticasone furoate/vilanterol (VI) combination in healthy subjects. Br J Clin Pharmacol 77:466–479
Worth H, Chung KF, Felser JM, Hu H, Rueegg P (2011) Cardio- and cerebrovascular safety of indacaterol vs formoterol, salmeterol, tiotropium and placebo in COPD. Respir Med 105:571–579
Covelli H, Bhattacharya S, Cassino C, Conoscenti C, Kesten S (2005) Absence of electrocardiographic findings and improved function with once-daily tiotropium in patients with chronic obstructive pulmonary disease. Pharmacotherapy 25:1708–1718
Lasseter KC, Aubets J, Chuecos F, Gil EG (2011) Aclidinium bromide, a long-acting antimuscarinic, does not affect QT interval in healthy subjects. J Clin Pharmacol 51:923–932
Acknowledgments
Editorial support in the form of development of a draft outline in consultation with the authors, development of a manuscript first draft in consultation with the authors, editorial suggestions to draft versions of this paper, assembling tables and collating author comments, copyediting, fact checking, and referencing was provided by Tara N Miller, PhD (employed by Gardiner-Caldwell Communications, Lyndhurst, NJ, USA, until November 2013) and by Laura Maguire, MChem, at Gardiner-Caldwell Communications (Gardiner-Caldwell Communications, Macclesfield, UK) and was funded by GSK. Editorial support in the form of styling the manuscript to comply with journal guidelines was provided by James Andrews, BA, of Fishawack Indicia Ltd and then in the form of peer-review comment incorporation by Joanne Ashworth, BSc, also of Fishawack Indicia Ltd and was also funded by GSK.
Funding
The study was sponsored by GSK. Employees of the sponsor were involved in the conception, design and conduct of the study, and in data collection and analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
RM and BP are employees of, and hold stock in, GSK. MG and JW were former employees of Pharsight, a contract research organization, which was contracted by GSK to perform the analyses presented in this manuscript; they did not receive any financial support for their involvement in development of the manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehta, R., Green, M., Patel, B. et al. Concentration-QT analysis of the randomized, placebo- and moxifloxacin-controlled thorough QT study of umeclidinium monotherapy and umeclidinium/vilanterol combination in healthy subjects. J Pharmacokinet Pharmacodyn 43, 153–164 (2016). https://doi.org/10.1007/s10928-015-9461-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-015-9461-x