Abstract
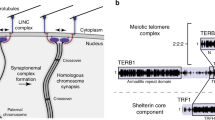
In eukaryotes, chromosome ends (telomeres) are tethered to the inner nuclear membrane. During the early stages of meiosis, telomeres move along the nuclear membrane and gather near the spindle-pole body, resulting in a bouquet-like arrangement of chromosomes. This chromosomal configuration appears to be widely conserved among eukaryotes, and is assumed to play an important role in the normal progression of meiosis, by mediating the proper pairing of homologous chromosomes. In fission yeast, the Bqt1–Bqt2 protein complex plays a key role in tethering the telomere to the inner nuclear membrane. However, the structural details of the complex required to clarify how telomeres are gathered near the spindle-pole body remain enigmatic. Previously, we devised a preparation procedure for the Schizosaccharomyces japonicus Bqt1–Bqt2 complex, in which a SUMO tag was fused to the N-terminus of the Bqt1 protein. This allowed us to purify the Bqt1–Bqt2 complex from the soluble fraction. In the present study, we found that a maltose-binding protein homolog, Athe_0614, served as a better fusion partner than the SUMO protein, resulting in the marked increase in the solubility of the Bqt1–Bqt2 complex. The Athe_0614 fusion partner may open up new avenues for X-ray crystallographic analyses of the structure of the Bqt1–Bqt2 complex.




Similar content being viewed by others
References
Scherthan H (2001) A bouquet makes ends meet. Nat Rev Mol Cell Biol 2:621–627
Chikashige Y, Ding DQ, Funabiki H et al (1994) Telomere-led premeiotic chromosome movement in fission yeast. Science 264:270–273
Ding DQ, Yamamoto A, Haraguchi T et al (2004) Dynamics of homologous chromosome pairing during meiotic prophase in fission yeast. Dev Cell 6:329–341
Chikashige Y, Haraguchi Y, Hiraoka Y (2007) Another way to move chromosomes. Chromosoma 116:497–505
Yamamoto A, Hiraoka Y (2001) How do meiotic chromosomes meet their homologous partners? lessons from fission yeast. Bioessays 23:526–533
Ding DQ, Haraguchi T, Hiraoka Y (2010) From meiosis to postmeiotic events: alignment and recognition of homologous chromosomes in meiosis. FEBS J 277:565–570
Cooper JP (2000) Telomere transitions in yeast: the end of the chromosome as we know it. Curr Opin Genet Dev 10:169–177
Chikashige Y, Tsutsumi C, Yamane M et al (2006) Meiotic proteins Bqt1 and Bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 125:59–69
Ichikawa Y, Kagawa W, Saito K et al (2013) Purification and characterization of the fission yeast telomere clustering factors, Bqt1 and Bqt2. Protein Expr Purif 88:207–213
Starr DA, Han M (2003) ANChors away: an actin based mechanism of nuclear positioning. J Cell Sci 116:211–216
Tzur YB, Wilson KL, Gruenbaum Y (2006) SUN-domain proteins: “Velcro” that links the nucleoskeleton to the cytoskeleton. Nat Rev Mol Cell Biol 7:782–788
Méjat A, Misteli T (2010) LINC complexes in health and disease. Nucleus 1:40–52
Hiraoka Y, Dernburg AF (2009) The SUN rises on meiotic chromosome dynamics. Dev Cell 17:598–605
Larkin MA, Blackshields G, Brown NP et al (2007) Clustal W and clustal version 2.0. Bioinformatics 23:2947–2948
Rhind N, Chen Z, Yassour M et al (2011) Comparative functional genomics of the fission yeasts. Science 332:930–936
Svoboda A, Bähler J, Kohli J et al (1995) Microtubule-driven nuclear movements and linear elements as meiosis-specific characteristics of the fission yeasts Schizosaccharomyces versatilis and Schizosaccharomyces pombe. Chromosoma 104:203–214
Kobe B, Ve T, Williams SJ (2015) Fusion-protein-assisted protein crystallization. Acta Cryst F71:861–869
Hoover DM, Lubkowski J (2002) DNAWorks: an automated method for designing oligonucleotides for PCR-based gene synthesis. Nucleic Acids Res 30:e43
Laue TM, Shah B, Ridgeway TM et al (1992). In: Harding SE, Rowe AJ, Horton LC (eds) Analytical ultracentrifugation in biochemistry and polymer science. Royal Society of Chemistry, Cambridge
Thorsen TS, Matt R, Weis WI et al (2014) Modified T4 lysozyme fusion proteins facilitate G protein-coupled receptor crystallogenesis. Structure 22:1657–1664
Ullah H, Scappini EL, Moon AF et al (2008) Structure of a signal transduction regulator, RACK1, from Arabidopsis thaliana. Protein Sci 17:1771–1780
Kobe B, Center RJ, Kemp BE et al (1999) Crystal structure of human T cell leukemia virus type 1 gp21 ectodomain crystallized as a maltose-binding protein chimera reveals structural evolution of retroviral transmembrane proteins. Proc Natl Acad Sci USA 96:4319–4324
Moon AF, Mueller GA, Zhong X et al (2010) A synergistic approach to protein crystallization: combination of a fixed-arm carrier with surface entropy reduction. Protein Sci 19:901–913
Huang DT, Hunt HW, Zhuang M et al (2007) Basis for a ubiquitin-like protein thioester switch toggling E1-E2 affinity. Nature 445:394–398
Yokoyama H, Yamashita T, Morioka R et al (2014) Extracellular secretion of noncatalytic plant cell wall-binding proteins by the cellulolytic thermophile Caldicellulosiruptor bescii. J Bacteriol 196:3784–3792
Deis LN, Wu Q, Wang Y et al (2015) Suppression of conformational heterogeneity at a protein–protein interface. Proc Natl Acad Sci USA 112:9028–9033
Yokoyama H, Yamashita T, Horikoshi N et al (2013) Crystallization and preliminary X-ray diffraction analysis of the secreted protein Athe_0614 from Caldicellulosiruptor bescii. Acta Cryst F69:438–440
Funding
This study was funded by JSPS KAKENHI Grant Numbers: JP19K12328, JP24570138, JP26116521 and JP16H01316 (to W.K.); JP18H05534 and JP17H01408 (to H.K.); JP18H05528 (to T.H.); JP18H05533 (to Y.H.). W.K. was also supported by the Science Research Promotion Fund from the Japan Private School Promotion Foundation, and by the Priority Research Funding from Meisei University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuzurihara, H., Aizawa, Y., Saotome, M. et al. Improved Methods for Preparing the Telomere Tethering Complex Bqt1–Bqt2 for Structural Studies. Protein J 39, 174–181 (2020). https://doi.org/10.1007/s10930-020-09887-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-020-09887-z