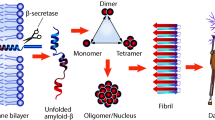
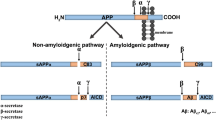
Abstract
Amyloid-β (Aβ) peptides are involved in Alzheimer’s disease (AD) development. The interactions of these peptides with copper and zinc ions also seem to be crucial for this pathology. Although Cu(II) and Zn(II) ions binding by Aβ peptides has been scrupulously investigated, surprisingly, this phenomenon has not been so thoroughly elucidated for N-truncated Aβ4−x—probably the most common version of this biomolecule. This negligence also applies to mixed Cu–Zn complexes. From the structural in silico analysis presented in this work, it appears that there are two possible mixed Cu–Zn(Aβ4−x) complexes with different stoichiometries and, consequently, distinct properties. The Cu–Zn(Aβ4−x) complex with 1:1:1 stoichiometry may have a neuroprotective superoxide dismutase-like activity. On the other hand, another mixed 2:1:2 Cu–Zn(Aβ4−x) complex is perhaps a seed for toxic oligomers. Hence, this work proposes a novel research direction for our better understanding of AD development.
Graphical Abstract




Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Maynard CJ, Bush AI, Masters CL et al (2005) Metals and amyloid-β in Alzheimer’s disease. Int J Exp Pathol 86:147–159. https://doi.org/10.1111/j.0959-9673.2005.00434.x
Walsh DM, Selkoe DJ (2020) Amyloid β-protein and beyond: the path forward in Alzheimer’s disease. Curr Opin Neurobiol 61:116–124. https://doi.org/10.1016/j.conb.2020.02.003
Drew SC (2017) The case for abandoning therapeutic chelation of copper ions in Alzheimer’s disease. Front Neurosci 11:317. https://doi.org/10.3389/fnins.2017.00317
Kepp KP (2016) Alzheimer’s disease due to loss of function: a new synthesis of the available data. Prog Neurobiol 143:36–60. https://doi.org/10.1016/j.pneurobio.2016.06.004
Lei P, Ayton S, Bush AI (2021) The essential elements of Alzheimer’s disease. J Biol Chem 296:100105. https://doi.org/10.1074/jbc.REV120.008207
Faller P, Hureau C, Berthoumieu O (2013) Role of metal ions in the self-assembly of the Alzheimer’s amyloid-β peptide. Inorg Chem 52:12193–12206. https://doi.org/10.1021/ic4003059
Wolfe MS (2019) In search of pathogenic amyloid β-peptide in familial Alzheimer’s disease. In: Progress in Molecular Biology and Translational Science, 1st ed. Elsevier Inc., pp 71–78
Masters CL, Simms G, Weinman NA et al (1985) Amyloid plaque core protein in Alzheimer disease and down syndrome. Proc Natl Acad Sci U S A 82:4245–4249. https://doi.org/10.1073/pnas.82.12.4245
Miller DL, Papayannopoulos IA, Styles J et al (1993) Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer′s Disease. Arch Biochem Biophys 301:41–52
Naslund J, Schierhorn A, Hellman U et al (1994) Relative abundance of Alzheimer Aβ amyloid peptide variants in Alzheimer disease and normal aging. Proc Natl Acad Sci 91:8378–8382. https://doi.org/10.1073/pnas.91.18.8378
Lewis H, Beher D, Cookson N et al (2006) Quantification of Alzheimer pathology in ageing and dementia: Age-related accumulation of amyloid-β(42) peptide in vascular dementia. Neuropathol Appl Neurobiol 32:103–118. https://doi.org/10.1111/j.1365-2990.2006.00696.x
Portelius E, Bogdanovic N, Gustavsson MK et al (2010) Mass spectrometric characterization of brain amyloid beta isoform signatures in familial and sporadic Alzheimer’s disease. Acta Neuropathol 120:185–193. https://doi.org/10.1007/s00401-010-0690-1
Moore BD, Chakrabarty P, Levites Y et al (2012) Overlapping profiles of Abeta peptides in the Alzheimer’s disease and pathological aging brains. Alzheimers Res Ther 4:18. https://doi.org/10.1186/alzrt121
Fritschi SK, Langer F, Kaeser SA et al (2014) Highly potent soluble amyloid-β seeds in human Alzheimer brain but not cerebrospinal fluid. Brain 137:2909–2915. https://doi.org/10.1093/brain/awu255
Portelius E, Lashley T, Westerlund A et al (2015) Brain amyloid-beta fragment signatures in pathological ageing and Alzheimer’s disease by hybrid immunoprecipitation mass spectrometry. Neurodegener Dis 15:50–57. https://doi.org/10.1159/000369465
Wirths O, Walter S, Kraus I et al (2017) N-truncated Aβ4−x peptides in sporadic Alzheimer’s disease cases and transgenic Alzheimer mouse models. Alzheimer’s Res Ther 9:80. https://doi.org/10.1186/s13195-017-0309-z
Wildburger NC, Esparza TJ, Leduc RD et al (2017) Diversity of amyloid-beta proteoforms in the Alzheimer’s disease brain. Sci Rep 7:9520. https://doi.org/10.1038/s41598-017-10422-x
Cabrera E, Mathews P, Mezhericher E et al (2018) Aβ truncated species: implications for brain clearance mechanisms and amyloid plaque deposition. Biochim Biophys Acta - Mol Basis Dis 1864:208–225. https://doi.org/10.1016/j.bbadis.2017.07.005
Gkanatsiou E, Portelius E, Toomey CE et al (2019) A distinct brain beta amyloid signature in cerebral amyloid angiopathy compared to Alzheimer’s disease. Neurosci Lett 701:125–131. https://doi.org/10.1016/j.neulet.2019.02.033
Zampar S, Klafki HW, Sritharen K et al (2020) N-terminal heterogeneity of parenchymal and vascular amyloid-β deposits in Alzheimer’s disease. Neuropathol Appl Neurobiol 46:673–685. https://doi.org/10.1111/nan.12637
Mukherjee S, Perez KA, Lago LC et al (2021) Quantification of N-terminal amyloid-β isoforms reveals isomers are the most abundant form of the amyloid-β peptide in sporadic Alzheimer’s disease. Brain Commun. https://doi.org/10.1093/braincomms/fcab028
Shen L, Ji HF (2007) Rat’s trick to escape Alzheimer’s disease. J Biomol Struct Dyn 25:271–273. https://doi.org/10.1080/07391102.2007.10507175
Gaggelli E, Janicka-Klos A, Jankowska E et al (2008) NMR studies of the Zn2+ interactions with rat and human β-amyloid (1–28) peptides in water-micelle environment. J Phys Chem B 112:100–109. https://doi.org/10.1021/jp075168m
Alies B, Borghesani V, Noël S et al (2018) Mutations of histidine 13 to arginine and arginine 5 to glycine are responsible for different coordination sites of zinc(II) to human and murine peptides. Chem A Eur J 24:14233–14241. https://doi.org/10.1002/chem.201802759
Wirths O, Zampar S, Weggen S (2019) N-terminally truncated Aβ peptide variants in Alzheimer’s Disease. In: Alzheimer’s disease. Codon Publications, pp 107–122
Tõugu V, Karafin A, Zovo K et al (2009) Zn(II)- and Cu(II)-induced non-fibrillar aggregates of amyloid-β (1–42) peptide are transformed to amyloid fibrils, both spontaneously and under the influence of metal chelators. J Neurochem 110:1784–1795. https://doi.org/10.1111/j.1471-4159.2009.06269.x
Noy D, Solomonov I, Sinkevich O et al (2008) Zinc-amyloid β interactions on a millisecond time-scale stabilize non-fibrillar Alzheimer-related species. J Am Chem Soc 130:1376–1383. https://doi.org/10.1021/ja076282l
Innocenti M, Salvietti E, Guidotti M et al (2010) Trace copper(II) or zinc(II) ions drastically modify the aggregation behavior of Amyloid-β1-42: An AFM study. J Alzheimer’s Dis 19:1323–1329. https://doi.org/10.3233/JAD-2010-1338
Bolognin S, Messori L, Drago D et al (2011) Aluminum, copper, iron and zinc differentially alter amyloid-Aβ1-42 aggregation and toxicity. Int J Biochem Cell Biol 43:877–885. https://doi.org/10.1016/j.biocel.2011.02.009
Sharma AK, Pavlova ST, Kim J et al (2013) The effect of Cu2+ and Zn2+ on the Aβ42 peptide aggregation and cellular toxicity. Metallomics 5:1529. https://doi.org/10.1039/c3mt00161j
Rezaei-Ghaleh N, Giller K, Becker S, Zweckstetter M (2011) Effect of zinc binding on β-amyloid structure and dynamics: Implications for Aβ aggregation. Biophys J 101:1202–1211. https://doi.org/10.1016/j.bpj.2011.06.062
Yang DS, McLaurin J, Qin K et al (2000) Examining the zinc binding site of the amyloid-β peptide. Eur J Biochem 267:6692–6698. https://doi.org/10.1046/j.1432-1327.2000.01767.x
Miura T, Suzuki K, Kohata N, Takeuchi H (2000) Metal binding modes of Alzheimer’s amyloid β-peptide in insoluble aggregates and soluble complexes. Biochemistry 39:7024–7031. https://doi.org/10.1021/bi0002479
Tsvetkov PO, Kulikova AA, Golovin AV et al (2010) Minimal Zn2+ binding site of amyloid-β. Biophys J 99:L84–L86. https://doi.org/10.1016/j.bpj.2010.09.015
Kozin SA, Mezentsev YV, Kulikova AA et al (2011) Zinc-induced dimerization of the amyloid-β metal-binding domain 1–16 is mediated by residues 11–14. Mol Biosyst 7:1053–1055. https://doi.org/10.1039/c0mb00334d
Alies B, Solari PL, Hureau C, Faller P (2012) Dynamics of ZnII binding as a key feature in the formation of amyloid fibrils by Aβ11-28. Inorg Chem 51:701–708. https://doi.org/10.1021/ic202247m
Alies B, LaPenna G, Sayen S et al (2012) Insights into the mechanisms of amyloid formation of ZnII -Ab11-28: pH-dependent zinc coordination and overall charge as key parameters for kinetics and the structure of ZnII -Ab11-28 aggregates. Inorg Chem 51:7897–7902. https://doi.org/10.1021/ic300972j
Syme CD, Viles JH (2006) Solution 1H NMR investigation of Zn2+ and Cd 2+ binding to amyloid-beta peptide (Aβ) of Alzheimer’s disease. Biochim Biophys Acta - Proteins Proteomics 1764:246–256. https://doi.org/10.1016/j.bbapap.2005.09.012
Talmard C, Bouzan A, Faller P (2007) Zinc binding to amyloid-β: Isothermal titration calorimetry and Zn competition experiments with Zn sensors. Biochemistry 46:13658–13666. https://doi.org/10.1021/bi701355j
Zirah S, Kozin SA, Mazur AK et al (2006) Structural changes of region 1–16 of the Alzheimer disease amyloid β-peptide upon zinc binding and in vitro aging. J Biol Chem 281:2151–2161. https://doi.org/10.1074/jbc.M504454200
Alies B, Conte-Daban A, Sayen S et al (2016) Zinc(II) binding site to the amyloid-β peptide: insights from spectroscopic studies with a wide series of modified peptides. Inorg Chem 55:10499–10509. https://doi.org/10.1021/acs.inorgchem.6b01733
Mekmouche Y, Coppel Y, Hochgräfe K et al (2005) Characterization of the ZnII binding to the peptide amyloid-β1-16 linked to Alzheimer’s disease. ChemBioChem 6:1663–1671. https://doi.org/10.1002/cbic.200500057
Danielsson J, Pierattelli R, Banci L, Gräslund A (2007) High-resolution NMR studies of the zinc-binding site of the Alzheimer’s amyloid β-peptide. FEBS J 274:46–59. https://doi.org/10.1111/j.1742-4658.2006.05563.x
Abelein A, Gräslund A, Danielsson J (2015) Zinc as chaperone-mimicking agent for retardation of amyloid β peptide fibril formation. Proc Natl Acad Sci 112:5407–5412. https://doi.org/10.1073/pnas.1421961112
Talmard C, Guilloreau L, Coppel Y et al (2007) Amyloid-beta peptide forms monomeric complexes with CuII and ZnII prior to aggregation. ChemBioChem 8:163–165. https://doi.org/10.1002/cbic.200600319
Branch T, Barahona M, Dodson CA, Ying L (2017) Kinetic analysis reveals the identity of Aβ-metal complex responsible for the initial aggregation of Aβ in the synapse. ACS Chem Neurosci 8:1970–1979. https://doi.org/10.1021/acschemneuro.7b00121
Istrate AN, Kozin SA, Zhokhov SS et al (2016) Interplay of histidine residues of the Alzheimer’s disease Aβ peptide governs its Zn-induced oligomerization. Sci Rep 6:21734. https://doi.org/10.1038/srep21734
Hori Y, Hashimoto T, Wakutani Y et al (2007) The Tottori (D7N) and English (H6R) familial Alzheimer disease mutations accelerate Aβ fibril formation without increasing protofibril formation. J Biol Chem 282:4916–4923. https://doi.org/10.1074/jbc.M608220200
Minicozzi V, Stellato F, Comai M et al (2008) Identifying the minimal copper- and zinc-binding site sequence in amyloid-β peptides. J Biol Chem 283:10784–10792. https://doi.org/10.1074/jbc.M707109200
Kwang HL, Yun KK, Chang YT (2007) Investigations of the molecular mechanism of metal-induced Aβ (1–40) amyloidogenesis. Biochemistry 46:13523–13532. https://doi.org/10.1021/bi701112z
Illes-Toth E, Meisl G, Rempel DL et al (2021) Pulsed hydrogen-deuterium exchange reveals altered structures and mechanisms in the aggregation of familial Alzheimer’s disease mutants. ACS Chem Neurosci 12:1972–1982. https://doi.org/10.1021/acschemneuro.1c00072
Radko SP, Khmeleva SA, Kaluzhny DN et al (2020) The english (H6R) mutation of the Alzheimer’s disease amyloid-β peptide modulates its zinc-induced aggregation. Biomolecules 10:961. https://doi.org/10.3390/biom10060961
Masters CL, Multhaup G, Simms G et al (1985) Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer’s disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J 4:2757–2763. https://doi.org/10.1002/j.1460-2075.1985.tb04000.x
Antonios G, Saiepour N, Bouter Y et al (2013) N-truncated Abeta starting with position four: early intraneuronal accumulation and rescue of toxicity using NT4X-167, a novel monoclonal antibody. Acta Neuropathol Commun 1:56. https://doi.org/10.1186/2051-5960-1-56
Sergeant N, Bombois S, Ghestem A et al (2003) Truncated beta-amyloid peptide species in pre-clinical Alzheimer’s disease as new targets for the vaccination approach. J Neurochem 85:1581–1591. https://doi.org/10.1046/j.1471-4159.2003.01818.x
Bouter Y, Noguerola JSL, Tucholla P et al (2015) Abeta targets of the biosimilar antibodies of Bapineuzumab, Crenezumab, Solanezumab in comparison to an antibody against N-truncated Abeta in sporadic Alzheimer disease cases and mouse models. Acta Neuropathol 130:713–729. https://doi.org/10.1007/s00401-015-1489-x
Shinohara M, Koga S, Konno T et al (2017) Distinct spatiotemporal accumulation of N-truncated and full-length amyloid-β42 in Alzheimer’s disease. Brain 140:3301–3316. https://doi.org/10.1093/brain/awx284
Bayer TA, Wirths O (2014) Focusing the amyloid cascade hypothesis on N-truncated Abeta peptides as drug targets against Alzheimer’s disease. Acta Neuropathol 127:787–801. https://doi.org/10.1007/s00401-014-1287-x
Luhrs T, Ritter C, Adrian M et al (2005) 3D structure of Alzheimer’s amyloid-β (1–42) fibrils. Proc Natl Acad Sci 102:17342–17347. https://doi.org/10.1073/pnas.0506723102
Cerofolini L, Ravera E, Bologna S et al (2020) Mixing Aβ(1–40) and Aβ(1–42) peptides generates unique amyloid fibrils. Chem Commun 56:8830–8833. https://doi.org/10.1039/d0cc02463e
Gremer L, Schölzel D, Schenk C et al (2017) Fibril structure of amyloid-β(1–42) by cryo–electron microscopy. Science 358:116–119. https://doi.org/10.1126/science.aao2825
Colvin MT, Silvers R, Ni QZ et al (2016) Atomic Resolution structure of monomorphic Aβ42 amyloid fibrils. J Am Chem Soc 138:9663–9674. https://doi.org/10.1021/jacs.6b05129
Xiao Y, Ma B, McElheny D et al (2015) Aβ(1–42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat Struct Mol Biol 22:499–505. https://doi.org/10.1038/nsmb.2991
Damante CA, Ösz K, Nagy Z et al (2011) Zn2+’s ability to alter the distribution of Cu2+ among the available binding sites of Aβ(1–16)-polyethylenglycol-ylated peptide: implications in Alzheimer’s disease. Inorg Chem 50:5342–5350. https://doi.org/10.1021/ic101537m
Alies B, Sasaki I, Proux O et al (2013) Zn impacts Cu coordination to amyloid-β, the Alzheimer’s peptide, but not the ROS production and the associated cell toxicity. Chem Commun 49:1214–1216. https://doi.org/10.1039/c2cc38236a
Mital M, Wezynfeld NE, Frączyk T et al (2015) A functional role for Aβ in metal homeostasis? N-truncation and high-affinity copper binding. Angew Chemie Int Ed 54:10460–10464. https://doi.org/10.1002/anie.201502644
Hahn M (1995) Receptor surface models. 1 definition and construction. J Med Chem 38:2080–2090. https://doi.org/10.1021/jm00012a007
Case DA, Cheatham TE, Darden T et al (2005) The Amber biomolecular simulation programs. J Comput Chem 26:1668–1688. https://doi.org/10.1002/jcc.20290
Maier JA, Martinez C, Kasavajhala K et al (2015) ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J Chem Theory Comput 11:3696–3713. https://doi.org/10.1021/acs.jctc.5b00255
Li Z, Song LF, Li P, Merz KM (2020) Systematic parametrization of divalent metal ions for the OPC3, OPC, TIP3P-FB, and TIP4P-FB water models. J Chem Theory Comput 16:4429–4442. https://doi.org/10.1021/acs.jctc.0c00194
Tsui V, Case DA (2000) Theory and applications of the generalized born solvation model in macromolecular simulations. Biopolymers 56:275–291. https://doi.org/10.1002/1097-0282(2000)56:4%3c275::AID-BIP10024%3e3.0.CO;2-E
Strange RW, Hough MA, Antonyuk SV, Hasnain SS (2012) Structural evidence for a copper-bound carbonate intermediate in the peroxidase and dismutase activities of superoxide dismutase. PLoS ONE 7:e44811. https://doi.org/10.1371/journal.pone.0044811
Kotuniak R, Strampraad MJF, Bossak-Ahmad K et al (2020) Key intermediate species reveal the copper(II)-exchange pathway in biorelevant ATCUN/NTS complexes. Angew Chemie Int Ed 59:11234–11239. https://doi.org/10.1002/anie.202004264
Pelmenschikov V, Siegbahn PEM (2005) Copper−zinc superoxide dismutase: theoretical insights into the catalytic mechanism. Inorg Chem 44:3311–3320. https://doi.org/10.1021/ic050018g
Tomaselli S, Esposito V, Vangone P et al (2006) The α-to-β conformational transition of Alzheimer’s Aβ-(1–42) peptide in aqueous media is reversible: a step by step conformational analysis suggests the location of β conformation seeding. ChemBioChem 7:257–267. https://doi.org/10.1002/cbic.200500223
Santoro A, Grimaldi M, Buonocore M et al (2021) Exploring the early stages of the amyloid Aβ(1–42) peptide aggregation process: an NMR study. Pharmaceuticals 14:732. https://doi.org/10.3390/ph14080732
Hu Z-W, Vugmeyster L, Au DF et al (2019) Molecular structure of an N-terminal phosphorylated β-amyloid fibril. Proc Natl Acad Sci 116:11253–11258. https://doi.org/10.1073/pnas.1818530116
Choi ES, Dokholyan NV (2021) SOD1 oligomers in amyotrophic lateral sclerosis. Curr Opin Struct Biol 66:225–230. https://doi.org/10.1016/j.sbi.2020.12.002
Jang J-Y, Cho H, Park H-Y et al (2017) ALS-linked mutant SOD1 proteins promote Aβ aggregates in ALS through direct interaction with Aβ. Biochem Biophys Res Commun 493:697–707. https://doi.org/10.1016/j.bbrc.2017.08.127
Takeda A, Tamano H (2015) Regulation of extracellular Zn2+ homeostasis in the hippocampus as a therapeutic target for Alzheimer’s disease. Expert Opin Ther Targets 19:1051–1058. https://doi.org/10.1517/14728222.2015.1029454
Huat TJ, Camats-Perna J, Newcombe EA et al (2019) Metal toxicity links to Alzheimer’s disease and neuroinflammation. J Mol Biol 431:1843–1868. https://doi.org/10.1016/j.jmb.2019.01.018
Frączyk T, Zawisza IA, Goch W et al (2016) On the ability of CuAβ1−x peptides to form ternary complexes: neurotransmitter glutamate is a competitor while not a ternary partner. J Inorg Biochem 158:5–10. https://doi.org/10.1016/j.jinorgbio.2016.02.035
Montoliu-Gaya L, Strydom A, Blennow K et al (2021) Blood biomarkers for Alzheimer’s disease in down syndrome. J Clin Med 10:3639. https://doi.org/10.3390/jcm10163639
Daniele S, Frosini D, Pietrobono D et al (2018) α-Synuclein heterocomplexes with β-amyloid are increased in red blood cells of parkinson’s disease patients and correlate with disease severity. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2018.00053
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
TF conceived, designed, performed the literature search and analysis. PC performed in silico analyses of the simulated structures. Both authors wrote the article.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frączyk, T., Cieplak, P. Neglected N-Truncated Amyloid-β Peptide and Its Mixed Cu–Zn Complexes. Protein J 41, 361–368 (2022). https://doi.org/10.1007/s10930-022-10056-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-022-10056-7