Abstract
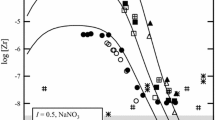
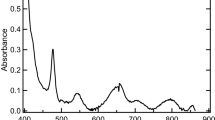
Chromium(III)-carbonate reactions are expected to be important in managing high-level radioactive wastes. Extensive studies on the solubility of amorphous Cr(III) hydroxide solid in a wide range of pH (3–13) at two different fixed partial pressures of CO2(g) (0.003 or 0.03 atm.), and as functions of K2CO3 concentrations (0.01 to 5.8 mol⋅kg−1) in the presence of 0.01 mol⋅dm−3 KOH and KHCO3 concentrations (0.001 to 0.826 mol⋅kg−1) at room temperature (22±2 °C) were carried out to obtain reliable thermodynamic data for important Cr(III)-carbonate reactions. A combination of techniques (XRD, XANES, EXAFS, UV-Vis-NIR spectroscopy, thermodynamic analyses of solubility data, and quantum mechanical calculations) was used to characterize the solid and aqueous species. The Pitzer ion-interaction approach was used to interpret the solubility data. Only two aqueous species [Cr(OH)(CO3) 2−2 and Cr(OH)4CO 3−3 ] are required to explain Cr(III)-carbonate reactions in a wide range of pH, CO2(g) partial pressures, and bicarbonate and carbonate concentrations. Calculations based on density functional theory support the existence of these species. The log 10 K° values of reactions involving these species [{Cr(OH)3(am) + 2CO2(g)⇌Cr(OH)(CO3) 2−2 +2H+} and {Cr(OH)3(am) + OH−+CO 2−3 ⇌Cr(OH)4CO 3−3 }] were found to be −(19.07±0.41) and −(4.19±0.19), respectively. No other data on any Cr(III)-carbonato complexes are available for comparisons.
Similar content being viewed by others
References
Fruchter, J.S.: In situ treatment of chromium-contaminated groundwater. Environ. Sci. Technol. 36, 464A–472A (2002)
Hrma, P., Vienna, J., Crum, J., Piepel, G., Mika, M.: Liquidus temperature of high-level waste borosilicate glasses with spinel primary phase. Mater. Res. Soc. Proc. 608, 671–676 (2000)
Swanson, J.L.: Detailed Description of First Example Flowsheet. Clean Option: An Alternative Strategy for Hanford Tank Waste Remediation, vol. 2. Pacific Northwest National Laboratory, Richland (1993)
Lumetta, G.J., Rapko, B.M.: Removal of chromium from Hanford tank sludges. Sep. Sci. Technol. 34, 1495–1506 (1999)
Rai, D., Hess, N.J., Rao, L., Zhang, Z., Felmy, A.R., Moore, D.A., Clark, S.B., Lumetta, G.J.: Thermodynamic model for the solubility of Cr(OH)3(am) in concentrated NaOH and NaOH-NaNO3 solutions. J. Solution Chem. 31, 343–367 (2002)
Rai, D., Moore, D.A., Hess, N.J., Rao, L., Clark, S.B.: Chromium(III) hydroxide solubility in the aqueous Na+-OH−-H2PO −4 -HPO 2−4 -PO 3−4 -H2O System: A thermodynamic model. J. Solution Chem. 33, 1213–1242 (2004)
Rai, D., Sass, B.M., Moore, D.A.: Cr(III) hydrolysis constants and solubility of Cr(III) hydroxide. Inorg. Chem. 26, 345–349 (1987)
Sass, B.M., Rai, D.: The solubility of amorphous Cr(III)-Fe(III) hydroxide solid solutions. Inorg. Chem. 26, 2228–2232 (1987)
Serne, R.J., Wyatt, G.A., Mattigod, S.V., Onishi, Y., Doctor, P.G., Bjornstad, B.N., Powell, M.R., Liljegren, L.M., Westsik, J., Aimo, N.J., Recknagle, K.P., Golcar, G.R., Miley, T.B., Holdren, G.R., Jeppson, D.W., Biyani, R.K., Barney., G.S.: Fluid Dynamic Particulate Segregation, Chemical Processes, Natural Ore Analog and Tank Inventory Discussions that Relate to the Potential for Criticality in Hanford Tanks. Westinghouse Hanford Company, Richland (1996)
Lindsay, W.L.: Chemical Equilibria in Soils. Wiley, New York (1979)
Weast, R.C.: Handbook of Chemistry and Physics, 53rd ed. The Chemical Rubber Company, Cleveland (1972)
Zhang, Z., Rao, L., Rai, D., Clark, S.B.: Characterization of chromium(III) hydroxide solids and their oxidation by hydrogen peroxide. Mater. Res. Soc. Symp. Proc. 824, CC6.5.1–CC6.5.6 (2004)
Wyckoff, R.W.G.: Crystal Structures. Krieger (1986)
Christensen, A.N., Hansen, P., Lehmann, M.S.: Isotope effects in the bonds of α-CrOOH and α-CrOOD. J. Solid State Chem. 21, 325–329 (1977)
Christensen, A.N., Hansen, P., Lehmann, M.S.: Isotope effects in the bonds of β-CrOOH and β-CrOOD. J. Solid State Chem. 19, 299–304 (1976)
Pitzer, K.S.: Ion Interaction Approach: Theory and Data Correlation. Chapter 3, Activity Coefficients in Electrolyte Solutions. CRC, Boca Raton (1991)
Pitzer, K.S.: Thermodynamics of electrolytes. I. Theoretical basis and general equations. J. Phys. Chem. 77, 268–277 (1973)
Sterner, S.M., Felmy, A.R., Rustad, J.R., Pitzer, K.S.: Thermodynamic Analysis of Aqueous Solutions Using INSIGHT. Pacific Northwest National Laboratory, Richland (1997)
Felmy, A.R., Rai, D., Schramke, J.A., Ryan, J.L.: The solubility of Pu(OH)3 in dilute solution and in high-ionic-strength chloride brines. Radiochim. Acta 48, 29–35 (1989)
Rai, D., Felmy, A.R., Sterner, S.M., Moore, D.A., Mason, M.J., Novak, C.F.: The solubility of Th(IV) and U(IV) hydrous oxides in concentrated NaCl and MgCl2 solutions. Radiochim. Acta 79, 239–247 (1997)
Rai, D., Felmy, A.R., Szelmeczka, R.W.: Hydrolysis constants and ion interaction parameters for Cd(II) in zero to high concentrations of NaOH, KOH, and the solubility product of crystalline Cd(OH)2. J. Solution Chem. 375, 375–390 (1991)
Rai, D., Xia, Y., Hess, N.J., Strachan, D.M., McGrail, B.P.: Hydroxo and chloro complexes/ion-interactions of Hf4+ and the solubility product of HfO2(am). J. Solution Chem. 30, 949–967 (2001)
Becke, A.D.: A new mixing of Hartree-Fock and local density functional theories. J. Chem. Phys. 98, 1372–1377 (1993)
Martin, R.L., Hay, P.J., Pratt, L.R.: Hydrolysis of ferric ion in water and conformational equilibrium. J. Phys. Chem. A 102, 3565–3573 (1998)
Rosso, K.M., Morgan, J.J.: Outer-sphere electron transfer kinetics of metal ion oxidation by molecular oxygen. Geochim. Cosmochim. Acta 66, 4223–4233 (2002)
Rosso, K.M., Rustad, J.R.: Ab initio calculation of homogeneous outer sphere electron transfer rates: Application to M(OH2) 3+/2+6 redox couples. J. Phys. Chem. A 104, 6718–6725 (2000)
Rosso, K.M., Smith, D.M.A., Dupuis, M.: Aspects of aqueous iron and manganese (II/III) self-exchange electron transfer reactions. J. Phys. Chem. 108, 5242–5248 (2004)
Apra, E., Bylaska, E.J., Jong, W.D., Hackler, M.T., Hirata, S., Pollack, L., Smith, D.M.A., Straatsma, T.P., Windus, T.L., Harrison, R.J., Nieplocha, J., Tipparaju, V., Kumar, M., Brown, E., Cisneros, G., Dupuis, M., Fann, G.I., Fruchtl, H., Garza, J., Hirao, K., Kendall, R., Nichols, J.A., Tsemekhman, K., Valiev, M., Wolinski, K., Anchell, J., Bernholdt, D., Borowski, P., Clark, T., Clerc, D., Dachsel, H., Deegan, M., Dyall, K., Elwood, D., Glendening, E., Gutowski, M., Hess, A., Jaffe, J., Johnson, B., Ju, J., Kobayashi, H., Kutteh, R., Lin, Z., Littlefield, R., Long, X., Meng, B., Nakajima, T., Niu, S., Rosing, M., Sandrone, G., Stave, M., Taylor, H., Thomas, G., Lenthe, J.V., Wong, A., Zhang, Z.: NWChem: A Computational Chemistry Package Designed to Run on High-Performance Parallel Supercomputers, Version 4.5. Pacific Northwest National Laboratory, Richland (2003)
Schafer, A., Huber, C., Ahlrichs, R.: Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 100, 5829–5835 (1994)
Krishnan, R., Binkley, J.S., Seeger, R., Pople, J.A.: Self-consistent molecular orbital methods. 20. Basis set for correlated wavefunctions. J. Chem. Phys. 72, 650–654 (1980)
Klamt, A.: Conductor-like screening model for real solvents—a new approach to the quantitative calculation of solvation phenomena. J. Phys. Chem. 99, 2224–2235 (1995)
Bylaska, E.J., Dixon, D.A., Felmy, A.R.: The free energies of reactions of chlorinated methanes with aqueous monovalent anions: Application of ab initio electronic structure theory. J. Phys. Chem. A 104, 610–617 (2000)
Teo, B.K.: Inorganic Chemistry Concepts 9: EXAFS: Basic Principles and Data Analysis. Springer, New York (1985)
Fujihara, T., Ichikawa, M., Gustafsson, T., Olovsson, I., Tsuchida, T.: Powder-neutron diffraction studies of geometric isotope and hydrogen-bonding effects in β-CrOOH. J. Phys. Chem. Solids 63, 309–315 (2002)
Corker, J.M., Evans, J., Rummey, J.M.: EXAFS studies of pillared clay catalysts. Mater. Chem. Phys. 29, 201–209 (1991)
Fendorf, S.E., Lamble, G.M., Stapleton, M.G., Kelley, M.J., Sparks, D.L.: Mechanisms of chromium(III) sorption on silica. 1. Cr(III) surface structure derived by extended absorption fine structure spectroscopy. Environ. Sci. Technol. 28, 284–289 (1994)
Harvie, C.E., Moller, N., Weare, J.: The prediction of mineral solubilities in natural waters: The Na-K-Mg-Ca-H-Cl-SO4-OH-HCO3-CO3-CO2-H2O system to high ionic strength at 25 °C. Geochim. Cosmochim. Acta 48, 723–751 (1984)
Rao, L., Rai, D., Felmy, A.R., Fulton, R.W.: Solubility of NaNd(CO3)2⋅6H2O in concentrated Na2CO3 and NaHCO3 solutions. Radiochim. Acta 75, 141–147 (1996)
Ziemniak, S.E., Jones, M.E., Combs, K.E.S.: Solubility and phase behavior of Cr(III) oxides in alkaline media at elevated temperatures. J. Solution Chem. 27, 33–66 (1998)
Richens, D.T.: The Chemistry of Aqua Ions. Wiley, New York (1997)
Swaddle, T.W., Rosenqvist, J., Yu, P., Bylaska, E., Phillips, B.L., Casey, W.H.: Kinetic evidence for five-coordination in AlOH(aq)(2+) ion. Science 308, 1450–1453 (2005)
Wesolowski, D.J.: Aluminum speciation and equilibria in aqueous solution: I. The Solubility of gibbsite in the system Na-K-Cl-OH-Al(OH)4 from 0 to 100 degrees C. Geochim. Cosmochim. Acta 56, 1065–1091 (1992)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rai, D., Moore, D.A., Hess, N.J. et al. Chromium(III) Hydroxide Solubility in the Aqueous K+-H+-OH−-CO2-HCO −3 -CO 2−3 -H2O System: A Thermodynamic Model. J Solution Chem 36, 1261–1285 (2007). https://doi.org/10.1007/s10953-007-9179-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-007-9179-5