Abstract
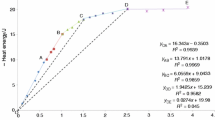
No thermodynamic data for Th complexes with aqueous Si are available. To obtain such data, extensive studies on ThO2(am) solubility were carried out as functions of: (1) a wide range of aqueous silica concentrations (0.0004 to 0.14 mol⋅L−1) at fixed pH values of about 10, 11, 12, and 13; and (2) and variable pH (ranging from 10 to 13.3) at fixed aqueous Si concentrations of about 0.006 mol⋅L−1 or 0.018 mol⋅L−1. The samples were equilibrated over long periods (ranging up to 487 days), and the data showed that steady-state concentrations were reached in < 29 days. X-ray diffraction, FTIR, and Raman analyses of the equilibrated solid phases showed that the Th solids were amorphous ThO2(am) containing some adsorbed Si. The solubility of ThO2(am) at pH values ranging from 10 to 13.3 at fixed 0.018 mol⋅L−1 aqueous Si concentrations decreases rapidly with an increase in pH, and increases dramatically with an increase in Si concentrations beyond about 0.003 mol⋅L−1 at fixed pH values > 10. The data were interpreted using both the Pitzer and SIT models, and required only the inclusion of one mixed-hydroxy-silica complex of Th [Th(OH)3(H3SiO4) 2−3 ]. Both models provided similar complexation constant values for the formation of this species. Density functional theory calculations predict complexes of this stoichiometry, having six-fold coordination of the Th cation, to be structurally stable. Predictions based on the fitted value of log 10 K 0=−18.5±0.7 for the ThO2(am) solubility reaction involving Th(OH)3(H3SiO4) 2−3 [ThO2(am)+3H4SiO4+H2O↔Th(OH)3(H3SiO4) 2−3 +2H+], along with the thermodynamic data for aqueous Si species reported in the literature, agreed closely with the extensive experimental data and showed that under alkaline conditions aqueous Si makes very strong complexes with Th.
Similar content being viewed by others
References
Rai, D., Yui, M., Hess, N.J., Felmy, A.R., Moore, D.A.: Thorium reactions in borosilicate-glass/water systems. Radiochim. Acta 93, 443–455 (2005). doi:10.1524/ract.2005.93.8.443
Peretroukhine, V., Riglet-Martial, C., Capdevila, H., Calmon, V., Bienvenu, P., Laszak, I.: Effect of soluble silicates on the solubility of thorium (IV) hydrous oxide. J. Nucl. Sci. Technol. Suppl. 3, 516–519 (2002)
Felmy, A.R.: A computerized chemical equilibrium model using a constrained minimization of the Gibbs free energy. PNL-728, Pacific Northwest National Laboratory, Richland (1990)
Sterner, S.M., Felmy, A.R., Rustad, J.R., Pitzer, K.S.: Thermodynamic analysis of aqueous solutions using INSIGHT. Pacific Northwest National Laboratory, Richland (1997)
Olin, A., Nolang, B., Osadchii, E.G., Rosen, E.: Chemical Thermodynamics of Selenium. Elsevier, Amsterdam (2005)
Pitzer, K.S.: Ion interaction approach: Theory and data correlation. In: Pitzer, K.S. (ed.) Activity Coefficients in Electrolyte Solutions, pp. 75–153. CRC Press, Boca Raton (1991)
Pitzer, K.S., Mayorga, G.: Thermodynamics of electrolytes. II. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J. Phys. Chem. 77, 2300–2308 (1973). doi:10.1021/j100638a009
Felmy, A.R., Weare, J.H.: The prediction of borate mineral equilibria in natural waters: Application to Searles lake, California. Geochim. Cosmochim. Acta 50, 2771–2783 (1986). doi:10.1016/0016-7037(86)90226-7
Felmy, A.R., Rai, D., Schramke, J.A., Ryan, J.L.: The solubility of Pu(OH)3 in dilute solution and in high-ionic-strength chloride brines. Radiochim. Acta 48, 29–35 (1989)
Grenthe, I., Fuger, J., Konings, R.J.M., Lemire, R.J., Muller, A.B., Nguyen-Trung, C., Wanner, H.: Chemical Thermodynamics of Uranium. North-Holland, Amsterdam (1992)
Felmy, A.R., Cho, H., Rustad, J.R., Mason, M.J.: An aqueous thermodynamic model for polymerized silica species to high ionic strength. J. Solution Chem. 30, 509–525 (2001). doi:10.1023/A:1010382701742
Klamt, A.: Conductor-like screening model for real solvents—A new approach to the quantitative calculation of solvation phenomena. J. Phys. Chem. 99, 2224–2235 (1995). doi:10.1021/j100007a062
Becke, A.D.: A new mixing of Hartree-Fock and local density functional theories. J. Chem. Phys. 98(2), 1372–1377 (1993). doi:10.1063/1.464304
Cao, X.Y., Dolg, M.: Relativistic energy-consistent ab initio pseudopotentials as tools for quantum chemical investigations of actinide systems. Coord. Chem. Rev. 250(7–8), 900–910 (2006)
Binkley, J.S., Pople, J.A., Hehre, W.J.: Self-consistent molecular orbital methods. 21. Small split-valence basis sets for 1st row elements. J. Am. Chem. Soc. 102(3), 939–947 (1980). doi:10.1021/ja00523a008
Tsushima, S., Yang, T., Mochizuki, Y., Okamoto, Y.: Ab initio study on the structures of Th(IV) hydrate and its hydrolysis products in aqueous solution. Chem. Phys. Lett. 375, 204–212 (2003). doi:10.1016/S0009-2614(03)00806-6
Apra, E., Windus, T.L., Straatsma, T.P., Bylaska, E.J., de Jong, W., Kowalski, K., Hackler, M.T., Hirata, S., Valiev, M., Hackler, M.T., Zhao, Y., Harrison, R.J., Dupuis, M., Smith, D.M.A., Nieplocha, J., Tipparaju, V., Krishnan, M., Auer, A.A., Brown, E., Cisneros, G., Fann, G.I., Fruchtl, H., Garza, J., Hirao, K., Kendall, R., Nichols, J.A., Tsemekhman, K., Wolinski, K., Anchell, J., Bernholdt, D., Borowski, P., Clark, T., Clerc, D., Dachsel, H., Deegan, M., Dyall, K., Elwood, D., Glendening, E., Gutowski, M., Hess, A., Jaffe, J., Johnson, B., Ju, J., Kobayashi, H., Kutteh, R., Lin, Z., Littlefield, R., Long, X., Meng, B., Nakajima, T., Niu, S., Pollack, L., Rosing, M., Sandrone, G., Stave, M., Taylor, H., Thomas, G., van Lenthe, J., Wong, A., Zhang, Z.: NWChem: A computational chemistry package designed to run on high-performance parallel supercomputers, Version 4.7. Pacific Northwest National Laboratory: Richland (2005)
Żegliński, J., Piotrowski, G.P., Piękoś, R.: A study of interaction between hydrogen peroxide and silica gel by FTIR spectroscopy and quantum chemistry. J. Mol. Struct. 794, 83–91 (2006). doi:10.1016/j.molstruc.2006.01.043
Nakamoto, K.: Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th edn. Wiley, New York (1986)
Hansen, H.C.B., Wetche, T.P., Raullund-Rasmussen, K., Borggaard, O.K.: Stability constants for silicate adsorbed to ferrihydrite. Clay Miner. 29, 341–350 (1994). doi:10.1180/claymin.1994.029.3.05
Kusabiraki, K., Shiraishi, Y.: Infrared spectrum of vitreous fayalite. J. Non-Cryst. Solids 44, 365–368 (1981). doi:10.1016/0022-3093(81)90038-7
Felmy, A.R., Rai, D., Sterner, S.M., Mason, M.J., Hess, N.J., Conradson, S.D.: Thermodynamic models for highly charged aqueous species: Solubility of Th(IV) hydrous oxide in concentrated NaHCO3 and Na2CO3 solutions. J. Solution Chem. 26, 233–248 (1997). doi:10.1007/BF02767996
Rai, D., Felmy, A.R., Moore, D.A., Mason, M.J.: The solubility of Th(IV) and U(IV) hydrous oxides in concentrated NaHCO3 and Na2CO3 solutions. In: Materials Research Society Symposium Proceedings (1995)
Ryan, J.L., Rai, D.: Thorium (IV). Hydrous oxide solubility. Inorg. Chem. 26, 4140–4142 (1987)
Brendebach, B., Altmaier, M., Rothe, J., Neck, V., Denecke, M.A.: EXAFS study of aqueous ZrIV and ThIV complexes in alkaline CaCl2 solutions: Ca3[Zr(OH)6]4+ and Ca4[Th(OH)8]4+. Inorg. Chem. 46, 6804–6810 (2007). doi:10.1021/ic070318t
Farges, F.: Structural environment around Th4+ in silicate glasses: Implications for the geochemistry of incompatible Me4+ elements. Geochim. Cosmochim. Acta 55, 3303–3319 (1991). doi:10.1016/0016-7037(91)90490-V
Wilson, R.E., Skanthakumar, S., Burns, P.C., Soderholm, L.: Structure of the homoleptic thorium(IV) aqua ion [Th(H2O)10]Br4. Angerwandte Chemie Int. Edn. 46, 8043–8045 (2007)
Wilson, R.E., Skanthakumar, S., Sigmon, G., Burns, P.C., Soderholm, L.: Structures of dimeric hydrolysis products of thorium. Inorg. Chem. 46, 2368–2372 (2007). doi:10.1021/ic0617691
Henning, C., Schmeide, K., Brendler, V., Moll, H., Tsushima, S., Scheinost, A.C.: EXAFS investigation of U(VI), U(IV), and Th(IV) sulfato complexes in aqueous solution. Inorg. Chem. 46, 5882–5892 (2007). doi:10.1021/ic0619759
Yang, T., Tsushima, S., Suzuki, A.: Quantum mechanical and molecular dynamical simulations on thorium(IV) hydrates in aqueous solution. J. Phys. Chem. A 105, 10439–10445 (2001). doi:10.1021/jp012387d
Okamoto, Y., Mochizuki, Y., Tsushima, S.: Theoretical study of hydrolysis reactions of tetravalent thorium ion. Chem. Phys. Lett. 373, 213–217 (2003). doi:10.1016/S0009-2614(03)00592-X
Azaroual, M., Fouillac, C., Matray, J.M.: Solubility of silica polymorphs in electrolyte solutions. 1. Activity coefficient of aqueous silica from 25 degrees to 250 degrees C, Pitzer’s parameterisation. Chem. Geol. 140, 155–165 (1997). doi:10.1016/S0009-2541(97)00046-6
Hershey, J.P., Millero, F.J.: The dependence of the acidity constants of silicic-acid on NaCl concentration using Pitzer equations. Mar. Chem. 18, 101–105 (1986). doi:10.1016/0304-4203(86)90079-4
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rai, D., Yui, M., Moore, D.A. et al. Thermodynamic Model for ThO2(am) Solubility in Alkaline Silica Solutions. J Solution Chem 37, 1725–1746 (2008). https://doi.org/10.1007/s10953-008-9344-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-008-9344-5