Abstract
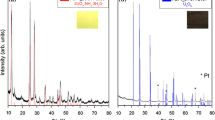
A 30 year-old PuF4 sample consisting of brown powder (PuF4-b) and pink granules (PuF4-p) was analyzed. X-ray diffraction shows the bulk is comprised of three compounds: PuF4, PuO2, and PuF4·1.6H2O. Broadening of PuF4 XRD peaks suggests possible \(\upalpha\)-damage. After annealing at 650 °C, crystalline PuF4 and PuO2 remain. Thermogravimetric analysis and differential scanning calorimetry—with simultaneous evolved gas analysis—of the separated PuF4-p and PuF4-b components reveal a distinct sequence of reactions. Dehydration occurs between ~ 90 and 300 °C. Exothermic annealing of the \(\upalpha\)-damage occurs in two stages: at 350–355 °C and at 555–558 °C. Hydrofluoric acid, fluorine and helium desorb during the first exotherm. Above 700 °C, PuF4 reacts with PuO2, resulting in oxygen release and mass loss.











Similar content being viewed by others
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical and time limitations.
References
Parker TG, Albrecht-Schmitt TE, Polinski MJ, Wang S, Diwu J (2019) In: Clark DL, Geeson DA, Hanrahan RJ (eds) The Plutonium Handbook, 2nd edn., vol 3, Ch. 19, 1419–1452, American Nuclear Society
Clark DL, Hecker SS, Jarvinen GD, Neu MP (2011) In: Morss LR, Edelstein NM, Fuger J, Katz JJ (eds.) The Chemistry of the Actinide and Transactinide Elements, 4th edn., vol 2, 1264 p., Springer: Dordrecht, Netherlands
McCoy KM, Casella A, Sinkov S, Sweet L, McNamara B, Delegard C, Jevremovic T (2017). J Nucl Mat. https://doi.org/10.1016/j.jnucmat.2017.08.005
Baker RD (1946) Los Alamos National Laboratory Unclassified Report, LA-473, 65 p
Conner WV (1966) Dow Chemical Company, Rocky Flats Division, RFP-728, 28 p.
Johns IB, Moulton GH (1944) Los Alamos National Laboratory Unclassified Report, LA-193, 20 p.
Johns IB (1945) Los Alamos National Laboratory Unclassified Report, LA-381, 11 p.
Moser WS, Navratil JD (1984) J Less Common Metals 100:171–187
Blankenship FF (1964) In: Molten salt reactor program semiannual progress Report, ORNL-3708, 252–287
Beneš O, Konings RJM (2009) J Fluorine Chem 130:22–29
Beneš O, Konings RJM (2012): Comp Nucl Mat, DOI https://doi.org/10.1016/B978-0-08-056033-5.00062-8
Molten Salt Panel of the Committee on Remediation of Buried and Tank Wastes (1997) Margrave JL (Chair) National Research Council National Academies Press, 148 p.
Peretz FJ, Rushton JE, Faulkner RL, Walker KL, DelCul GD (1998) ORNL-CP-98416, 9 p.
Bamberger CE, Ross RG, Baes CF Jr (1971) J Inorg Nucl Chem 33:767–776
Claquesin J, Lemoine O, Gibilaro M, Massot L, Chamelot P, Bourges G (2019). Electrochim Acta. https://doi.org/10.1016/j.electacta.2019.01.169
Paget T, McNeese J, Fife K, Jackson JM, Watson R (2019) In: Clark DL, Geeson DA, Hanrahan RJ (eds.) The Plutonium Handbook, 2 edn., 1–6, 201–286
Ensslin N (1991) In: Reilly D, Ensslin N, Smith Jr. H, Kreiner S (eds.) Passive nondestructive assay of nuclear materials, NUREG/CR-5550, LA-UR-90–0732
Narlesky JE, Stroud MA, Smith PH, Wayne DM, Mason RE, Worl LA (2012) Los Alamos National Laboratory Unclassified Report, LA-UR-12–23790 (2012) 114 p.
Tandon L (2000) Los Alamos National Laboratory Unclassified Report, LA-13725-MS (2000) 85 p.
Clark DL, Funk DJ (2015) Los Alamos National Laboratory Unclassified Report, LA-UR-15–22393, 64 p.
Smith DM, Neu MP, Garcia E, Morales LA (2000) Waste Manage 20:479–490
Crowder ML, Duffey JM, Livingston RR, Scogin JH, Kessinger GF, Almond PM (2009) J Alloys Comp 488:565–567
Wayne DM (2016) Los Alamos National Laboratory Unclassified Report, LA-UR-16–23438, 391 p.
Wayne DM, White JT (2019) In: Clark DL, Geeson DA, Hanrahan RJ (eds.) The Plutonium Handbook, 2nd edn., 6–44, 3167–3200
Colletti LP (2019) In: Clark DL, Geeson DA, Hanrahan RJ (eds.) The Plutonium Handbook, 2nd edn., 6–45, 3420–3434
Hj M (1992) Nucl Inst Methods Phys Res B 65:30–39
Ellsworth S, Navrotsky A, Ewing RC (1994) Phys Chem Mineral 21:140–149
Farnan I, Cho H, Weber WJ (2007) Nature 445:190–193
Wiss T, Hiernaut JP, Roudil D, Colle JY, Maugeri E, Talip Z, Janssen A, Rondinella V, Konings RJM, Matzke Hj, Weber WJ (2014) J Nucl Mat 451, 198–206
Simeone D, Costantini JM, Luneville L, Desgranges L, Trocellier P, Garcia P (2015) J Mater Res 30:1495–1515
Weber WJ, Wald JW, Matzke Hj (1985) Mat Res Soc Symp Proc 44, 679–686
Wronkiewicz DJ (1993) In: MRS Proceedings, Scientific Basis for Nuclear Waste Management, DOI https://doi.org/10.1557/PROC-333-083
Bower WR, Pearce CI, Droop GTR, Mosselmans JFW, Geraki K, Pattrick RAD (2015) Mineral Mag DOI https://doi.org/10.1180/minmag.2015.079.6.20
Lumpkin GR (2001) J Nucl Mat 289:136–166
Groote JC, Seinen J, Weerkamp JRW, Den Hartog HW (1991), Rad Eff Def Solids, DOI https://doi.org/10.1080/10420159108220844
Dubinko VI, Turkin AA, Vainshtein DI, den Hartog HW (1999). J Appl Phys. https://doi.org/10.1063/1.371639
den Hartog HW, Vainshtein DI, Dubinko VI, Turkin AA (2002) Nucl Inst Methods Phys Res B 191:168–172
Exarhos GJ (1982) J Phys Chem 86:4020–4025
Luo JS, Liu GK (2001) J Mater Res 16:366–372
Pei Y, Chen X, Yao D, Ren G (2007) Rad Meas 42:407–412
Danišík M, McInnes BIA, Kirkland CL, McDonald BJ, Evans NJ, Becker T (2017). Sci Adv. https://doi.org/10.1126/sciadv.1601121
Schwartz AJ, Wall MA, Zocco TG, Wolfer WG (2005). Phil Mag. https://doi.org/10.1080/02678370412331320026
Jeffries JR, Hammons JA, Willey TM, Wall MA, Ruddle D, Ilavsky J, Allen PG, van Buuren T (2018) J Nucl Mat 498:505–510
Ronchi C, Hiernaut JP (2004) J Nucl Mat 325:1–12
Reiners PW (2005) Rev Mineral Geochem 58:151–179
Flowers RM, Ketcham RA, Shuster DL, Farley KA (2009) Geochim Cosmochim Acta 73:2347–2365
Guenthner WR, Reiners PW, Ketcham RA, Nasdala L, Geister G (2013). Am J Sci. https://doi.org/10.2475/03.2013.01
Baughman JS, Flowers RM, Metcalf JR, Dhansay T (2017) Geochim Cosmochim Acta 205:50–64
Ginster U, Reiners PW, Nasdala L, Chanmuang NC (2019) Geochim Cosmochim Acta 249:225–246
Ault AK, Guenthner WR, Moser AC, Miller GH, Refsnider KA (2018) Chem Geol 490:1–12
Dawson JK, Elliott RM, Hurst R, Truswell AE (1954) J Chem Soc, 558–564
Dawson JK, D'Eye WM, Truswell AE (1954) J Chem Soc, 3922–3929
Dawson JK, Elliott RM (1953), Great Britain AERE Report, AERE-C/R-1207, Harwell, UK, 25 p.
Claux B, Beneš O, Capelli E, Souček P, Meier R (2016) J Fluorine Chem 183:10–13
Tosolin A, Souček P, Beneš O, Vigier J-F, Luzzi L, Konings RJM (2018) J Nucl Mat 503:171–177
Chudinov ÉG (1970) Choporov D Ya. Atomnaya Énergiya 28:151–153
Casella A, Carter J, Lines A, Bello J, Bryan S, Clark R, Corbey J, Delegard C, Heller F, McNamara B, Sweet L (2019) Actinide Research Quarterly, No. 1, 2nd Quarter, 31–35
Katz JJ, Sheft I (1960) In: Advances in inorganic chemistry and radiochemistry, Vol. 2, DOI https://doi.org/10.1016/S0065-2792(08)60190-9
Fried S, Davidson NR (1947) Unclassified Report (June 30) United States Atomic Energy Commission, Oak Ridge, TN., MDDC-1250, 11 p.
Luerkens DW (1984) Savannah River Laboratory Unclassified Report DPST-83–1065 35 p.
Martella LL, Saba MT, Campbell GK (1984) Rockwell International, Rocky Flats Plant, RFP-3589, 22 p.
Sohn CL, Thorn CW, Christensen DC (1982) 6th Actinide Separation Workshop, Augusta, GA, May 11–12, LA-UR-82–1230, 28p
Christensen DC, Rayburn JA (1983) Los Alamos National Laboratory Report LA-9655-MS, UC-10, 11 p
Morgan Jr. AN, Baker RD, Hazen WC, Henrickson AV, McNeese WD, Thomas RL (1958), In: 2nd United Nations International Conference on the Peaceful Uses of Atomic Energy, A/CONF.15/P/531, 11 July, 19 p.
Brunauer S, Emmett PH, Teller E (1938) J Am Chem Soc 60:309–319
Balzar D (1999) In: Snyder RL, Fiala J, Bunge HJ (eds.); Defect and microstructure analysis by diffraction; international union of crystallography: Chester, England, 1999; p 94
ASTM E 967–08 (2014) ASTM International, United States
ASTM E 1582–10 (2010) ASTM International, United States
Haire RG, (2019) In: Clark DL, Geeson DA, Hanrahan RJ (eds) The Plutonium Handbook, 2nd edn., V. 3, Ch. 20, 1453–1490, American Nuclear Society
Gabbott P (2008). In: Gabbott P (ed) Principles and applications of thermal analysis. Blackwell, Oxford UK, pp 1–50
Wiss T, Dieste-Blanco O, Tacu A, Janssen A, Talip Z, Colle J-Y, Martin P, Konings R (2015) J Mater Res 30:1544–1554
Myers N (1956) USAEC Rept. HW-45128, Hanford Atomic Products Operation, General Electric Co., 31 p.
Orr RM, Sims HE, Taylor RJ (2015) J Nucl Mat 465:756–773
Murakami T, Chakoumakos BC, Ewing RC, Lumpkin GR, Weber WJ (1991) Am Mineral 76:1510–1532
Westrum Jr., EF, Wallman AC (1950) Lawrence Berkeley National Laboratory Report, UCRL-697, 6 p.
Oetting FL (1967) Chem Rev 67:261–297
Cleveland, JM (1980) In: Wick OJ (ed.) Plutonium Handbook A Guide to the Technology, V. 1, American Nuclear Society, p 335–402.
Rand MH, Fuger J (2000) European Commission Joint Research Centre, EUR 17332, 96 p.
Barton CJ, Strehlow, (1961) J. lnorg. Nucl Chem 18:143–147
Mulford RNR (1993) Los Alamos National Laboratory Unclassified Report LA-12569, UC-701, 24 p.
Glushko VP, Gurvich LV, Bergman GA, Veits IV, Medvedev VA, Khachkuruzov GA, Yungman VS (1982) Thermodynamic properties of individual substances IV. Nauka, Moscow
Asker WJ, Wylie AW (1965) Aust J Chem 18:969–975
Chatain S, Morel B (2016) In: Reference module in materials science and materials engineering, DOI https://doi.org/10.1016/B978-0-12-803581-8.00674-3 12016
Acknowledgements
We thank 2 anonymous reviewers, and Mr. Gary Sevigny, and Drs. Sergey I. Sinkov, T. Gannon Parker and two anonymous Journal reviewers for reviewing this paper and suggesting useful to this manuscript. Dr. Steve Yarbro provided valuable input pertaining to the origin of the LANL PuF4.
Funding
This work was supported by the US Department of Energy through the Los Alamos National Laboratory. Los Alamos National Laboratory is operated by Triad National Security, LLC, for the National Nuclear Security Administration of U.S. Department of Energy (Contract No. 89233218CNA000001). This work was supported by the NA-22 Office of the US Department of Energy.
Author information
Authors and Affiliations
Contributions
Dr. David M. Wayne: conceptualization, methodology, investigation, formal analysis, resources, validation, data curation, writing (original draft), writing (review & editing), visualization. Dr. Jared Stritzinger: formal analysis, validation, data curation, resources, writing (review & editing), visualization. Dr. Amanda J. Casella: formal analysis, validation, data curation, resources, writing (review & editing). Dr. Lucas E. Sweet: investigation, data curation, writing. Dr. Jordan F. Corbey: investigation, data curation, writing. Mr. Daniel J. Garcia: investigation. Dr. E. Miller Wylie: investigation. Dr. Lav Tandon: funding acquisition, supervision, project administration, resources, writing (review & editing). Dr. Angela C. Olson: supervision, project administration, writing (review & editing), resources. Dr. Jung Ho Rim: investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or proprietary interests in any material discussed in this article.
Human and animal testing
No animal or human testing was performed for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wayne, D.M., Stritzinger, J.T., Casella, A.J. et al. X‐ray diffraction, differential scanning calorimetry and evolved gas analysis of aged plutonium tetrafluoride (PuF4). J Radioanal Nucl Chem 329, 741–756 (2021). https://doi.org/10.1007/s10967-021-07810-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07810-z