Abstract
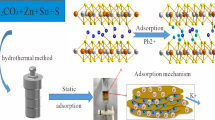
A novel metal sulfide NaTS-2 was prepared using a simple one-step hydrothermal method to remove strontium ions from the simulated radioactive wastewater, in which metal tin was replaced by tin salts to reduce the cost of the raw materials. NaTS-2 has a layered structure with an approximate molar ratio of Na:Sn:S of 1.94:2.87:7. NaTS-2 could rapidly remove Sr2+ with a removal efficiency of 99.3% ± 0.23% within 1 min and at an equilibrium of 60 min, followed by the pseudo-second-order model and Langmuir model with a maximum adsorption capacity of 88.9 ± 0.81 mg/g at 298 K. NaTS-2 exhibited an outstanding affinity for Sr2+ (Kd ≥ (3.0 ± 0.10) × 105 mL/g) over a broad pH range of 3–11, also displayed significant affinity (Kd ≥ (1.0 ± 0.15) × 105 mL/g) and excellent removal efficiency (≥ 98.5% ± 0.48%) for typical activated corrosion products (Co2+, Mn2+ and Ni2+). NaTS-2 is a promising adsorbent in the field of radioactive wastewater treatment.













Similar content being viewed by others
References
Zhang X, Gu P, Liu Y (2019) Decontamination of radioactive wastewater: State of the art and challenges forward. Chemosphere 215:543–553
Li X, Liu Z, Huang M (2022) Purification of uranium-containing wastewater by adsorption: a review of research on resin materials. J Radioanal Nucl Chem 331:3043–3075
Asahara A, Kawasaki D, Yanagihara S (2021) Study on strategy construction for dismantling and radioactive waste management at Fukushima Daiichi Nuclear Power Station. Nucl Eng Des 374:111066
Le QT, Cho K (2021) Caesium adsorption on a zeolitic imidazolate framework (ZIF-8) functionalized by ferrocyanide. J Colloid Interface Sci 581:741–750
Jin X, Gu P, Zhang G, Shang X, Hou L (2014) Removal of nickel and strontium from simulated radioactive wastewater via a pellet coprecipitation-microfiltration process. J Radioanal Nucl Chem 301:513–521
Zhang X, Liu Y (2020) Ultrafast removal of radioactive strontium ions from contaminated water by nanostructured layered sodium vanadosilicate with high adsorption capacity and selectivity. J Hazard Mater 398:122907
Eun S, Ryu J, Kim H, Hong H, Kim S (2021) Simultaneous removal of radioactive cesium and strontium from seawater using a highly efficient Prussian blue-embedded alginate aerogel. J Environ Manage 297:113389
Su T, Han Z, Qu Z, Chen Y, Lin X, Zhu S, Bian R, Xie X (2020) Effective recycling of Co and Sr from Co/Sr-bearing wastewater via an integrated Fe coagulation and hematite precipitation approach. Environ Res 187:109654
Hodkin DJ, Stewart DI, Graham JT, Burke IT (2016) Coprecipitation of 14C and Sr with carbonate precipitates: the importance of reaction kinetics and recrystallization pathways. Sci Total Environ 562:335–343
Warrant RW, Reynolds JG, Johnson ME (2012) Removal of 90Sr and 241Am from concentrated Hanford chelate-bearing waste by precipitation with strontium nitrate and sodium permanganate. J Radioanal Nucl Chem 295:1575–1579
Zhang Y, Chong JY, Xu R, Wang R (2021) Effective separation of water-DMSO through solvent resistant membrane distillation (SR-MD). Water Res 197:117103
Ali S, Shah IA, Huang H (2020) Selectivity of Ar/O2 plasma-treated carbon nanotube membranes for Sr (II) and Cs (I) in water and wastewater: Fit-for-purpose water treatment. Sep Purif Technol 237:116352
Wu L, Cao J, Wu Z, Zhang J, Yang Z (2017) The mechanism of radioactive strontium removal from simulated radioactive wastewater via a coprecipitation microfiltration process. J Radioanal Nucl Chem 314:1973–1981
Wang W, Luo J, Wei W, Liu S, He J, Ma J (2021) An asymmetric pulsed current-assisted electrochemical method for Sr Sr (II) extraction using supramolecular composites. Chemosphere 271:129531
Momen MA, Dietz ML (2021) Extraction chromatographic materials based on polysulfone microcapsules for the sorption of strontium from aqueous solution. React Funct Polym 160:104829
Sharma JN, Khan PN, Dhami PS, Jagasia P, Tessy V, Kaushik CP (2019) Separation of strontium-90 from a highly saline high level liquid waste solution using 4, 4′ (5′) -[di-tert-butyldicyclohexano]-18-crown-6+ isodecyl alcohol/n-dodecane solvent. Sep Purif Technol 229:115502
Dai Q, Zhang W, Dong F, Zhao Y (2014) Effect of γ-ray radiation on the biosorption of strontium ions to baker’s yeast. Chem Eng J 249:226–235
Shukla A, Parmar P, Saraf M (2017) Radiation, radionuclides and bacteria: an in-perspective review. J Environ Radioact 180:27–35
Ngwenya N, Chirwa EM (2011) Biological removal of cationic fission products from nuclear wastewater. Water Sci Technol 63:124–128
Huo J, Yu G, Wang J (2021) Adsorptive removal of Sr(II) from aqueous solution by polyvinyl alcohol/graphene oxide aerogel. Chemosphere 278:130492
Alby D, Charnay C, Heran M, Prelot B, Zajac J (2018) Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: synthesis and shaping, sorption capacity, mechanisms, and selectivity—A review. J Hazard Mater 344:511–530
Kamble P, Roy PS, Banerjee D, Ananthanarayanan A, Shah JG, Sugilal G, Agarwal K (2019) A new composite of crystalline silicotitanate for sequestration of 137Cs and 90Sr from low-level aqueous waste solution. Sep Sci Technol 55:1603–1610
Chen Z, Wu Y, Wei Y, Mimura H (2015) Preparation of silica-based titanate adsorbents and application for strontium removal from radioactive contaminated wastewater. J Radioanal Nucl Chem 307:931–940
Zhang M, Gu P, Dong L (2017) Application of novel adsorption materials in 90Sr-contaminated wastewater treatment. Huagong Jinzhan 36(11):4142–4150
Yang D, Zheng Z, Zhu H, Liu H, Gao X (2008) Titanate nanofibers as intelligent absorbents for the removal of radioactive ions from water. Adv Mater 20:2777–2781
Liang C, Jia M, Du Z, Wang X, Men J, Hu J (2020) Research progress of the separation of strontium by adsorption material. Chem Mater 48(2):25–31
Zhang Z, Zhang M, Gu P, Zhang G (2019) Progress in adsorption of radioactive strontium and cesium from aqueous solution on zeolite materials. Huagong Jinzhan 38(4):1984–1995
Li X, Liu B, Jian Y, Zhong W, Mu W, He J, Ma Z, Liu G, Luo S (2012) Ion-exchange characteristics of a layered metal sulfide for removal of Sr2+ from aqueous solutions. Sep Sci Technol 47:896–902
Liang C, Jia M, Wang X, Du Z, Men J, Ding H (2019) Preparation of potassium niobium sulfide and its selective adsorption properties for Sr2+ and Co2+. J Radioanal Nucl Chem 322:377–387
Makhova L, Mikhlin Y, Romanchenko A (2007) A combined XPS, XANES and STM/STS study of gold and silver deposition on metal sulphides. Nucl Instrum Meth A 575:75–77
Dafauti S, Pulhani V, Hegde AG (2012) Applicability of layered metal sulphide for estimation of Sr concentration in groundwater. Desalin Water Treat 38:264–270
Zhang Z, Gu P, Zhang M, Yan S, Dong L, Zhang G (2019) Synthesis of a robust layered metal sulfide for rapid and effective removal of Sr2+ from aqueous solutions. Chem Eng J 372:1205–1215
Qi X, Du K, Feng M, Li J, Du C, Zhang B, Huang X (2015) A two-dimensionally microporous thiostannate with superior Cs+ and Sr2+ ion-exchange property. J Mater Chem 3:5665–5673
Sarma D, Malliakas CD, Subrahmanyam KS, Islam SM, Kanatzidid MG (2016) K2xSn4−xS8−x (x= 0.65–1): a new metal sulfide for rapid and selective removal of Cs+, Sr2+ and UO22+ ions. Chem Sci 7(2):1121–1132
Manos MJ, Kanatzidis MG (2009) Highly efficient and rapid Cs+ uptake by the layered metal sulfide K2xMnxSn3−xS6 (KMS-1). J Am Chem Soc 131:6599–6607
Mertz JL, Fard ZH, Malliakas CD, Manos MJ, Kanatzidis MG (2013) Selective removal of Cs+, Sr2+, and Ni2+ by K2xMgxSn3–xS6 (x = 0.5–1) (KMS-2) relevant to nuclear waste remediation. Chem Mater 25:2116–2127
Zhang M, Gu P, Zhang Z, Liu J, Dong L, Zhang G (2018) Effective, rapid and selective adsorption of radioactive Sr2+ from aqueous solution by a novel metal sulfide adsorbent. Chem Eng J 351:668–677
Zhang M, Gu P, Yan S, Dong L, Zhang G (2020) Na/Zn/Sn/S (NaZTS): Quaternary metal sulfide nanosheets for efficient adsorption of radioactive strontium ions. Chem Eng J 379:122227
Zhang W, Wang L (2014) Adsorption and desorption capacities for methylene blue with the cellulose-based nano-composite. Ind Water Treat 34(5):45–49
Hyung H, Kim JH (2008) Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: Effect of NOM characteristics and water quality parameters. Environ Sci Technol 42:4416–4421
Apul OG, Wang Q, Zhou Y, Karanfil T (2013) Adsorption of aromatic organic contaminants by graphene nanosheets: comparison with carbon nanotubes and activated carbon. Water Res 47:1648–1654
Cheng W, Dastgheib SA, Karanfil T (2005) Adsorption of dissolved natural organic matter by modified activated carbons. Water Res 39:2281–2290
Chung HK, Kim WH, Park J, Cho J (2015) Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J Ind Eng Chem 28:241–246
Zhao Z (2005) Application principle of adsorption. J Eng Therm Energy Power 20(6):631–631
Dong C, Deng X, Guo X, Wang B, Ye X, Fan J, Zhu C, Fan F, Qing B (2021) Synthesis of potassium metal ferrocyanide/Al-MCM-41 with fast and selective adsorption of cesium. Colloid Surface A 613:126107
Tran HN, You SJ, Hosseini-Bandegharaei A, Chao HP (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116
Faghihian H, Iravani M, Moayed M, Ghannadi-Maragheh M (2013) Preparation of a novel PAN-zeolite nanocomposite for removal of Cs+ and Sr2+ from aqueous solutions: kinetic, equilibrium, and thermodynamic studies. Chem Eng J 222:41–48
Mortazavian S, An H, Chun D, Moon J (2018) Activated carbon impregnated by zero-valent iron nanoparticles (AC/NZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: Material characterizations and kinetic studies. Chem Eng J 353:781–795
Zhang Y, Lin X, Hu S, Zhang X, Luo X (2016) Core–shell zeolite@alg–Ca particles for removal of strontium from aqueous solutions. RSC Adv 6:73959–73973
Li W, Li J, Zhang B (2021) Layered thiostannates with distinct arrangements of mixed cations for the selective capture of Cs+, Sr2+, and Eu3+ ions. ACS Appl Mater Interfaces 13(8):10191–10201
Mu W, Du S, Li X, Yu Q, Hu R, Wei H, Yang Y, Peng S (2019) Efficient and irreversible capture of strontium ions from aqueous solution using metal-organic frameworks with ion trapping groups. Dalton Trans 48:3284–3290
Acknowledgements
The authors are grateful for the financial support from the Major Science and Technology Program for Water Pollution Control and Treatment of China (2015ZX07406006), and Foundation of Tianjin University (2202).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, J., Wang, Z., Zhang, G. et al. Rapid and effective removal of strontium ions from aqueous solutions by a novel layered metal sulfide NaTS-2. J Radioanal Nucl Chem 332, 2367–2378 (2023). https://doi.org/10.1007/s10967-023-08850-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08850-3