Abstract
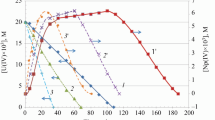
The process of chemical uranium enrichment by light isotopes has been studied. The kinetic parameters of the adsorption of UO2(NO3)2·2TBP molecules in a heterogeneous system at the vertical interface between a polar HNO3 solution and a solid vertical surface of nonpolar polypropylene (C3H6)n during the extraction of uranyl nitrate UO2(NO3)2 with tributylphosphate from an HNO3 solution were measured.














Similar content being viewed by others
References
Laskarin BN, Babenko AM, Filippov EA (1975) Chemical methods for separation of uranium isotopes. Usp Khim 156(5):761–781
Woodard RW (1957) Isotope exchange process. Patent US, No. 2787587
Maomi S, Tetsuya M, Kunihiko T (1973) Redox uranium isotope separation using anon exchangers. Patent US, No. 4,118,457
Kawasaki TM, Yokohama KT, Fujisawa HO (1983) Ion exchange enrichment of uranium isotopes. Patent US, No. 4,368,175
Zhiganov AN, Kondakov VM, Korotkevich VM (1998) Method for chemical separation of uranium isotopes. Patent RU 2,120,329
Delval P (1981) Method for isotopic chemical enrichment of uranium. Patent RU 867,283
Velavendan P, Ganesh S, Pandey K, Geetha R, Ahmed MK, Kamachi Mudali U, Natarajan R (2013) Studies on solubility of TBP in aqueous solutions of fuel reprocessing. J Radioanal Nucl Chem 295:1113–1117
Kramarenko EYu, Gordievskaya YuD (2017) Principles of self-organization in solutions of amphiphilic molecules. Russian Academy of Sciences, Moscow
Pavlyuchenko MM, Lazerko GA (1954) Kinetics of cadmium chloride ammonia formation. J Phys Chem 28(1):102–108
Erofeev BV, Sokolova ND (1963) Tables for calculations according to the topokinetic equation α-1-exp (-ktn). Academy of Sciences of the BSSR, Minsk
Sakovich GV (1955) Remarks on some equations of reactions kinetics involving solids currently in use. Scientific notes of V.V. Kuibyshev Tomsk State University 26:103–110
Douven S, Paez CA, Gommes CJ (2015) The range of validity of sorption kinetic models. J Colloid Interface Sci 448:437–450
Korzh EA, Klimenko NA (2017) Modeling the kinetics of adsorption of pharmaceutical substances on active carbons. Probl Mod Sci Educ 87(5):1–7
Krizhanovskaya OO, Sinyaeva LA, Karpov SI (2014) Kinetic models describing the sorption of fat-soluble physiologically active substances by highly ordered inorganic silicon-containing polymers. Sorpt Chromatogr Process 15(5):784–794
Ho YS, McKay G (1998) Kinetic Models for Sorption of Dye from Aqueous Solution by Wood. Process Saf Environ Prot 76:183–191
Ho YS, McKay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76:332–340
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater V.B136:681–689
Yakout SM (2010) Batch kinetics, isotherm and thermodynamic studies of adsorption of strontium from aqueous solutions onto low cost rice-straw based carbons. Carb Sci Technol 1:144–153
Zinovyev VG, Serebrov AP (2015) Evaluation of natural radioactivity levels for structural material used in construction of the neutrino detector. World J Nucl Sci Technol 5:43–56
Firestone RB (1998) Table of isotopes. Lawrence Berkeley National Laboratory, California. CD-ROM
Gromov BV (1978) Introduction to chemical technology of uranium, 1st edn. Atomizdat, Moscow
Loginova AYu, Gerasimova NS (2008) Chemical kinetics. University (MSTU), Moscow, N.E. Bauman Tech
Emanuel NM, Knorre DG (1984) Coursebook of chemical kinetics, 4th edn. Higher school, Moscow
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zinoviev, V.G., Rumyantseva, D.A., Serebrov, A.P. et al. Chemical uranium enrichment by light isotopes in a (C3H6)n-TBP-HNO3 heterogeneous system. J Radioanal Nucl Chem 332, 2027–2038 (2023). https://doi.org/10.1007/s10967-023-08883-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08883-8