Abstract
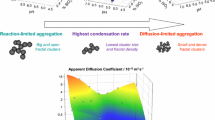
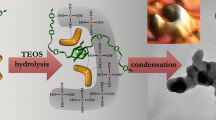
Silica matrices synthesized from a pre-hydrolysis step in ethanol followed by alcohol removal at low pressure distillation, and condensation in water, are suitable for encapsulation of biomolecules and microorganisms and building bioactive materials with optimized optical properties. Here we analyze the microstructure of these hydrogels from the dependence of I(q) data acquired from SAXS experiments over a wide range of silica concentration and pH employed in the condensation step. From the resulting data it is shown that there is a clear correlation between the microscopic parameters—cluster fractal dimension (D), elementary particle radius (a) and cluster gyration radius (R)—with the attenuation of visible light when the condensation step proceeds at pH < 6. At higher pHs, there is a steep dependence of the cluster density (~R D−3) with the condensation pH, and non-monotonous changes of attenuance are less than 20%, revealing the complexity of the system. These results, which were obtained for a wide pH and silica concentration range, reinforce the idea that the behavior of gels determined in a restricted interval of synthesis variables cannot be extrapolated, and comparison of gelation times is not enough for predicting their properties.






Similar content being viewed by others
References
Brinker CJ, Scherer G (1990) Sol gel science. Academic Press, San Diego
Gill I, Ballesteros A (1998) Encapsulation of biologicals within silicate, siloxane, and hybrid sol–gel polymers: an efficient and generic approach. J Am Chem Soc 120(34):8587–8598
Avnir D, Brown S, Lev O, Ottolenghi M (1994) Enzymes and other proteins entrapped in sol–gel materials. Chem Mater 6(10):1605–1614
Avnir D, Lev O, Livage J (2006) Recent bio-applications of sol-gel materials. J Mater Chem 16(11):1013–1030
Livage J, Coradin T (2006) Living cells in oxide glasses. Rev Mineral Geochem 64(1):315–332
Soltmann U, Böttcher H (2008) Utilization of sol-gel ceramics for the immobilization of living microorganisms. J Sol-Gel Sci Technol 342:211
Meunier CF, Dandoy P, Su B-L (2010) Encapsulation of cells within silica matrixes: towards a new advance in the conception of living hybrid materials. J Colloid Interface Sci 48:66–72
Premkumar JR, Lev O, Marks RS, Polyak B, Rosen R, Belkin S (2001) Antibody-based immobilization of bioluminescent bacterial sensor cells. Talanta 55(5):1029–1038
Perullini M, Rivero MM, Jobbagy M, Mentaberry A, Blimes SA (2007) Plant cell proliferation inside an inorganic host. J Biotechnol 127(3):542–548
Fiedler D, Hager U, Franke H, Soltmann U, Böttcher H (2007) Algae biocers: Astaxanthin formation in sol-gel immobilised living microalgae. J Mater Chem 17(3):261–266
Kuncova G, Podrazky O, Ripp S, Trögl J, Sayler GS, Demnerova K, Vankova R (2004) Monitoring of the viability of cells immobilized by sol–gel process. J Sol–Gel Sci Technol 31:1–8
Nguyen-Ngoc H, Durrieu C, Tran-Minh C (2009) Synchronous-scan fluorescence of algal cells for toxicity assessment of heavy metals and herbicides. Ecotoxicol Environ Saf 72:316–320
Sicard C, Perullini M, Spedalieri C, Coradin T, Brayner R, Livage J, Jobbagy M, Bilmes SA (2011) CeO2 nanoparticles for the protection of photosynthetic organisms immobilized in silica gels. Chem Mater 23(6):1374–1378
Perullini M, Jobbágy M, Bermúdez Moretti M, Correa García S, Bilmes SA (2008) Optimizing silica encapsulation of living cells: in situ evaluation of cellular stress. Chem Mater 20:3015–3018
Brumberger H (ed) (1993) Modern aspects of small-angle scattering.In: Proceedings of the NATO advanced study institutes, Como, Italy
Schmidt PW, Höhr A, Neumann H-B, Kaiser H, Avnir D, Lin JS (1989) Small angle X-ray scattering study of the fractal morphology of porous silicas. J Chem Phys 90(9):5016–5023
Vollet DR, Donatti DA, Ibãez Ruiz A, De Vicente FS (2010) Dynamical scaling in fractal structures in the aggregation of tetraethoxysilane-derived sonogels. J Appl Cryst 43(5):949–954
Zarzycki J (1987) Fractal properties of gels. J Non-Cryst Solids 95–96(1):173–184
Schaefer DW, Keefer KD (1984) Fractal geometry of silica condensation polymers. Phys Rev Lett 53(14):1383–1386
Mandelbrot BB (1983) The fractal geometry of nature. Freeman, San Francisco
Kim S, Lee K-S, Zachariah MR, Lee D (2010) Three-dimensional off-lattice Monte Carlo simulations on a direct relation between experimental process parameters and fractal dimension of colloidal aggregates. J Colloid Interface Sci 344:353–361
Schaefer DW, Martin JE, Wiltzius P, Cannell DS (1984) Fractal geometry of colloidal aggregates. Phys Rev Lett 52(26):2371–2374
Vollet DR, Donatti DA, Ibãez Ruiz A (2001) A SAXS study of kinetics of aggregation of TEOS-derived sonogels at different temperatures. J Non-Cryst Solids 288(1–3):81–87
Brinker CJ, Keefer KD, Schaefer DW, Assink RA, Kay BD, Ashley CS (1984) Sol gel transition in simple silicates. J Non-Cryst Solids 63:45–59
Strawbridge I, Craievich AF, James PF (1985) The effect of the H2O/TEOS ratio on the structure of gels derived by the acid catalysed hydrolysis of tetraethoxysilane. J Non-Cryst Solids 72:139–157
Himmel B, Gerberb T, Bürger H (1990) WAXS- and SAXS-investigations of structure formation in alcoholic SiO2 solutions. J Non-Cryst Solids 119:1–13
Reichenauer G (2004) Thermal aging of silica gels in water. J Non-Cryst Solids 350:189–195
Bhatia RB, Brinker CJ, Gupta AK, Singh AK (2000) Aqueous sol-gel process for protein encapsulation. Chem Mater 12:2434–2441
Coiffier A, Coradin T, Roux C, Bouvet O, Livage J (2001) Sol-gel encapsulation of bacteria: a comparison between alkoxide and aqueous routes. J Mater Chem 11:2039–2044
Nassif N, Roux C, Coradin T, Rager MN, Bouvet O, Livage J (2003) A sol-gel matrix to preserve the viability of encapsulated bacteria. Mater Chem 13:203–208
Gerberb T, Himmel B, Bürger H (1994) WAXS- and SAXS-investigations of structure formation of gels from sodium water glass. J Non-Cryst Solids 175:160–168
Perullini M, Amoura M, Roux C, Coradin T, Livage J, Japas ML, Jobbagy M, Bilmes SA (2011) Improving silica matrices for encapsulation of Escherichia coli using osmoprotectors. J Mater Chem 21:4546–4552
Ferrer ML, Del Monte F, Levy D (2002) A novel and simple alcohol-free sol-gel route for encapsulation of labile proteins. Chem Mater 14:3619–3621
Ferrer ML, Yuste L, Rojo F, Del Monte F (2003) Biocompatible sol-gel route for encapsulation of living bacteria in organically modified silica matrixes. Chem Mater 15:3614–3618
Ferrer ML, García-Carbajal ZY, Yuste L, Rojo F, Del Monte F (2006) Bacteria viability in sol–gel materials revisited: cryo-SEM as a suitable tool to study the structural integrity of encapsulated bacteria. Chem Mater 18:1458–1463
Cavalcanti LP, Torriani IL, Plivelic TS, Oliveira CLP, Kellermann G, Neuenschwander R (2004) Rev Sci Instrum 75:4541
Vinogradova E, Moreno A, Lara VH, Bosch P (2003) Multi-fractal imaging and structural investigation of silica hydrogels and aerogels. Silicon Chem 2:247–254
Vinogradova E, Moreno A, Lara VH, Bosch P (2003) Multi-fractal imaging and structural investigation of silica hydrogels and aerogels. Silicon Chem 2:247–254
Avnir D, Biham O, Lidar D, Malcai O (1998) Is the geometry of nature fractal? Science 279:39–40
Sorensen CM, Wang GM (1999) Size distribution effect on the power law regime of the structure factor of fractal aggregates. Phys Rev E 60(6):7143–7148
Knoblich B, Gerber T (2001) Aggregation in SiO2 sols from sodium silicate solutions. J Non Cryst Solids 283:109–113
Boukari H, Harris MT (1997) Small-angle X-ray scattering study of the formation of colloidal silica particles from alkoxides: primary particles or not? J Colloid Interface Sci 194:311–318
Knoblich B, Gerber T (2001) The arrangement of fractal clusters dependent on the pH value in silica gels from sodium silicate solutions. J Non Cryst Solids 296:81–87
Beelen TPM, Wijnen PWJG, Vonk CG, Van Santen RA (1989) Catal Lett 3:209
Bohren CG, Huffmann DF (1983) Absorption and scattering of light by small particles. Wiley, New York
Acknowledgements
This work has been supported by the Brazilian Synchrotron Light Laboratory (LNLS, Brazil, proposal D11A-SAXS-6039), the scientific collaboration agreement CAPES-SECyT (Brazil-Argentina, 011/02), the University of Buenos Aires (UBACyT X-003), and by National Research Council of Argentina (CONICET PIP 11220080102533). SAB, RC, MJ and MP are Research Scientists of CONICET (Argentina).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Perullini, M., Jobbágy, M., Bilmes, S.A. et al. Effect of synthesis conditions on the microstructure of TEOS derived silica hydrogels synthesized by the alcohol-free sol–gel route. J Sol-Gel Sci Technol 59, 174–180 (2011). https://doi.org/10.1007/s10971-011-2478-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-011-2478-8