Abstract
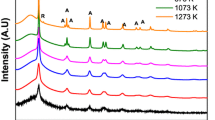
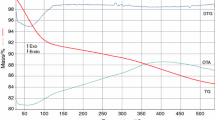
Silica-based mixed oxide xerogels, namely SiO2–CrO3, SiO2–MoO3, and SiO2–WO3, were prepared using the non-hydrolytic sol–gel process. The materials were synthesized using metal chloride:tetraethoxysilane (TEOS) molar ratios of 0.1:2; 0.2:2 and 0.4:2 for each metal chloride and 1:2 SiCl4:TEOS molar ratio. All of the xerogels containing Cr, Mo or W had considerably greater surface areas than that of SiO2. The small angle X-ray scattering experiments suggest that the surface roughness of the aggregates in SiO2–CrO3 is less than that of SiO2–MoO3 and SiO2–WO3. The morphological characteristics of the silica-based mixed oxide xerogels were not affected by the nature and amount of metal chloride employed in the synthesis. An irregular morphology was observed for SiO2–CrO3, SiO2–MoO3 and SiO2–WO3, but a lamellar structure was observed for SiO2. X-ray photoelectron spectroscopy analysis suggests that tungsten species were preferentially distributed on the outmost part of the grain. The resulting particle diameter was shown to be lower for the mixed oxides compared to that of bare silica. Furthermore, the presence of metals (Cr, Mo and W) on silica caused a decrease in the size of the particles as the atomic radii of these metals increased. According to the Fourier transform infrared spectroscopy and Raman, Cr, Mo and W were incorporated within the silica framework.












Similar content being viewed by others
References
Wachs IE, Roberts CA (2010) Monitoring surface metal oxide catalytic active sites with Raman spectroscopy. Chem Soc Rev 39:5002–5017
Arzoumanian H, Castellanos NJ, Martinez FO, Páez-Mozo EA, Ziarelli F (2010) Silicon-assisted direct covalent grafting on metal oxide surfaces: synthesis and characterization of carboxylate N, N′-ligands on TiO2. Eur J Inorg Chem 11:1633–1641
Irurzun VM, Tan Y, Resasco DE (2009) Sol–gel synthesis and characterization of Co–Mo/Silica catalysts for single-walled carbon nanotube production. Chem Mater 21(11):2238–2246
Gao G, Feng W, Wu G, Shen J, Zhang Z, Jin X, Zhang Z, Du A (2012) An investigation on the assembling of WO3 particles on the matrix of silica solution. J Sol–Gel Sci Technol 64:427–435
Wachs IE (2005) Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials. Catal Today 100:79–94
De Boer M, Van Dillen AJ, Koningsberger DC, Geus JW, Vuurman MA, Wachs IE (1991) Remarkable spreading behavior of molybdena on silica catalysts. An in situ EXAFS-Raman study. Catal Lett 11:227–240
Van Schalkwyk C, Spamer A, Moodley DJ, Dube T, Reynhardt J, Botha JM (2003) Application of a WO3/SiO2 catalyst in an industrial environment: part I. Appl Catal A 255:121–131
Choi KY, Tang S (2004) Polymerization rate modeling of ethylene polymerization with supported chromium oxide catalysts. J Appl Polym Sci 91:2923–2927
Li W, Meitzner GD, Borry RW III, Iglesia E (2000) Raman and X-ray absorption studies of Mo species in Mo/H-ZSM5 catalysts for non-oxidative CH4 reactions. J Catal 191:373–383
Thielemann JP, Ressler T, Walter A, Tzolova-Müller G, Hess C (2011) Structure of molybdenum oxide supported on silica SBA-15 studied by Raman, UV–Vis and X-ray absorption spectroscopy. Appl Catal A 399:28–34
Vioux A (1997) Nonhydrolytic sol–gel routes to oxides. Chem Mater 9:2292–2299
Mutin PH, Vioux A (2009) Nonhydrolytic processing of oxide-based materials: simple routes to control homogeneity, morphology, and nanostructure. Chem Mater 21:582–596
Niederberger M, Garnweitner G (2006) Organic reaction pathways in the nonaqueous synthesis of metal oxide nanoparticles. Chem Eur J 12:72827302
Debecker DP, Mutin PH (2012) Non-hydrolytic sol–gel routes to heterogeneous catalysts. Chem Soc Rev 41:3624–3650
Fisch AG, Cardozo NSM, Secchi AR, Stedile FC, Radtke C, de Sá DS, da Rocha ZN, dos Santos JHZ (2009) Immobilization of zirconocene within silica-tungsten by entrapment: Tuning electronic effects of the support on the supported complex. Appl Catal A 370:114–122
Fisch AG, Petry CF, Pozebon D, Stedile FC, Cardozo NSM, Secchi AR, dos Santos JHZ (2006) Immobilization of zirconocene into silica prepared by non-hydrolytic sol–gel method. Macromol Symp 245–246:77–86
Fisch AG, Cardozo NSM, Secchi AR, Stedile FC, Livotto PR, de As DS, da Rocha ZN, dos Santos JHZ (2009) Immobilization of metallocene within silica-titania by a non-hydrolytic sol–gel method. Appl Catal A 354:88–101
Fisch AG, Cardozo NSM, Secchi AR, Stedile FC, da Silveira NP, dos Santos JHZ (2008) Investigation of silica particle structure containing metallocene immobilized by a sol–gel method. J Non Cryst Solids 354:3973–3979
Hay JN, Haval HM (1998) Preparation of inorganic oxides via a non-hydrolytic sol–gel route. J Sol–Gel Sci Technol 13:109–112
Bourget L, Corriu RJP, Leclereq D, Mutin PH, Vioux A (1998) Non-hydrolytic sol–gel routes to silica. J Non Cryst Solids 242:81–91
Ilavsky J, Jemian PR (2009) Irena: tool suite for modeling and analysis of small-angle scattering. J Appl Crystallogr 42:347–353
Beaucage G (1995) Approximations leading to a unified exponential/power-law approach to small-angle scattering. Appl Crystallogr 28:717–728
Beaucage G (1996) Small-angle scattering from polymeric mass fractals of arbitrary mass-fractal dimension. Appl Crystallogr 29:134–146
Cullity BD (1978) Elements of X-ray diffraction, 2nd edn. Addison-Wesley Publishing Company Inc., Indiana, pp 1–509
Fidalgo A, Ilharco LM (2004) Chemical tailoring of porous silica xerogels: local structure by vibrational spectroscopy. Chem Eur J 10(2):392–398
Cappeletti LB, Moncada E, Poisson J, Butler I, dos Santos JHZ (2013) Determination of the network structure of sensor materials prepared by three different sol–gel routes using Fourier transform infrared spectroscopy (FT-IR). Appl Spectrosc 67(4):441
Ressler T (1998) High-pressure EXAFS and EDXRD K investigation of unit cell parameters of SnO. J Synch Rad 5:118
Newville M (2001) IFEFFIT: interactive EXAFS analysis and FEFF fitting. J Synch Rad 8:322–324
Mustre de Leon J, Rehr JJ, Zabinsky SI, Albers RC (1991) Ab initio curved-wave X-ray-absorption fine structure. Phys Rev B 44:4146–4156
Silveira F, Brambilla R, da Silveira NP, do Alves MCM, Stedile FC, Pergher SBC, dos Santos JHZ (2010) Effect of textural characteristics of supported metallocenes on ethylene polymerization. J Mater Sci 45:1760–1768
Fisch AG, Da Silveira N Jr, Cardozo NSM, Secchi AR, dos Santos JHZ, Soares JBP (2013) Direct production of ultra-high molecular weight polyethylene with oriented crystalline microstructures. J Mol Catal A: Chem 366:74–83
Wooster NZ (1930) International center for diffraction data. Kristall 74:363
Almeida RM (2004) Handbook of sol–gel science and technology: processing, characterization and applications. Volume II: characterization of sol–gel materials and products. Kluwer Academic Press, New York
Almeida RM, Guiton TA, Pantano CG (1990) Detection of LO Mode in ν-SiO2 by infrared diffuse reflectance spectroscopy. J Non Cryst Solids 119(2):238–241
Fidalgo A, Ciriminna R, Ilharco LM, Pagliaro M (2005) Role of the alkyl–alkoxide precursor on the structure and catalytic properties of hybrid sol–gel catalysts. Chem Mater 17(26):6686–6694
Morais EC, Correa GG, Brambilla R, Radtke C, Baibich IM, dos Santos JHZ (2013) The interaction of encapsulated pharmaceutical drugs with a silica matrix. Colloids Surf B Biointerfaces 103:422–429
Lana SLB, Seldon AB (1998) X-ray diffraction studies of sol–gel derived ORMOSILs based on combinations of tetramethoxysilane and trimethoxysilane. J Sol–Gel Sci Technol 13(1–3):461–466
Lee EL, Wachs IE (2007) In situ spectroscopic investigation of the molecular and electronic structures of SiO2 supported surface metal oxides. J Phys Chem C 111:14410–14425
Borghesi A, Guizzetti G, Marabelli F, Nosenzo L, Reguzzoni E (1984) Far-infrared optical properties of CrC13 and CrBr 3. Solid State Commun 52(4):463–465
Zhang Q, Wang Y, Ohishi Y, Shihido T, Takehira K (2001) Vanadium-containing MCM-41 for partial oxidation of lower alkanes. J Catal 202:308–318
Takehira K, Ohishi Y, Shishido T, Kawabata T, Takaki K, Zhang QH, Wang Y (2004) Behavior of active sites on Cr-MCM-41 catalysts during the dehydrogenation of propane with CO2. J Catal 224:404–416
Groppo E, Lamberti C, Bordiga S, Spoto G, Zecchina A (2005) The structure of active centers and the ethylene polymerization mechanism on the Cr/SiO2 catalyst: a frontier for the characterization methods. Chem Rev 105:115–184
Naydenov V, Tosheva L, Sterte J (2002) Spherical silica macrostructures containing vanadium and tungsten oxides assembled by the resin templating method. Microp Mosop Mat 55:253–263
Tromp M, Moulin J, Reid G, Evans J (2007) Cr K-Edge XANES spectroscopy: ligand and oxidation state dependence—what is oxidation state? AIP Conf Proc 882:699–701
Acknowledgments
This project was partially financed by CNPq and FAPERGS. A. Bernardes thanks CAPES for the Grant, and the authors are thankful to the LNLS (Project D11A-SAXS1-8691) for the measurements at the SAXS beamline and (Project XAFS1-9929) for the measurements at the EXAFS beamline.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bernardes, A.A., Radtke, C., Alves, M.C.M. et al. Synthesis and characterization of SiO2–CrO3, SiO2–MoO3, and SiO2–WO3 mixed oxides produced using the non-hydrolytic sol–gel process. J Sol-Gel Sci Technol 69, 72–84 (2014). https://doi.org/10.1007/s10971-013-3188-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-013-3188-1