Abstract
In this work, we carried out the synthesis of lead(II) divanadate(V) by means of a soft chemistry reaction based on a sol–gel-derived route. The final organic precursor was heat treated (T = 400, 500, 600, 750 and 800 °C) and structurally analyzed for each temperature by taking into account the results of FTIR spectroscopy, synchrotron X-ray powder diffraction and X-ray absorption near-edge structure. As an overall result, we report a final compound with remarkable crystallographic and morphological qualities that seem to keep all its structural features in the temperature range 450–700 °C before the structure incongruently melts. As a highlight, the desired material was obtained following a highly reproducible, low-cost, low-temperature and quite straightforward chemical route. Besides, this synthesis route could also allow the appropriate integration of lead(II) divanadate(V) nanoparticles, or nanolayers, into more complex systems as well as the feasibility for being expanded to other materials.
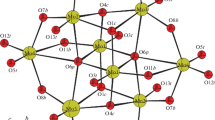
Graphical Abstract






Similar content being viewed by others
References
Blonska-Tabero A, Bosacka M (2013) Comparative studies in subsolidus areas of ternary oxide systems PbO–V2O5–In2O3 and PbO–V2O5–Fe2O3. J Therm Anal Calorim 113(1):137–145. doi:10.1007/s10973-013-2996-4
Bosacka M (2012) New indium lead(II) vanadate(V) in Pb2V2O7–InVO4 system and its characterization. J Alloy Compd 542:228–231. doi:10.1016/j.jallcom.2012.07.030
Zhou W, Tan D, Xiao W, Song M, Chen M, Xiong X, Xu J (2012) Structural properties of PbVO3 perovskites under hydrostatic pressure conditions up to 10.6 GPa. J phys Condens matter Inst Phys J 24(43):435403. doi:10.1088/0953-8984/24/43/435403
Martin LW, Zhan Q, Suzuki Y, Ramesh R, Chi M, Browning N, Mizoguchi T, Kreisel J (2007) Growth and structure of PbVO3 thin films. Appl Phys Lett 90(6):062903. doi:10.1063/1.2435944
Blonska-Tabero A, Filipek E (2014) New solid solution Pb2−xSrxFeV3O11—Synthesis, homogeneity range and characterization. J Alloy Compd 587:148–152. doi:10.1016/j.jallcom.2013.10.144
Guskos N, Typek J, Zolnierkiewicz G, Szymczak R, Berczynski P, Wardal K, Blonska-Tabero A (2011) Magnetic properties of a new iron lead vanadate Pb2FeV3O11. J Alloy Compd 509(32):8153–8157. doi:10.1016/j.jallcom.2011.05.114
Errandonea D, Popescu C, Achary SN, Tyagi AK, Bettinelli M (2014) In situ high-pressure synchrotron X-ray diffraction study of the structural stability in NdVO4 and LaVO4. Mater Res Bull 50:279–284. doi:10.1016/j.materresbull.2013.10.047
Goutaudier C, Ermeneux FS, Cohen-Adad MT, Moncorgé R, Bettinelli M, Cavalli E (1998) LHPG and flux growth of various Nd:YVO4 single crystals: a comparative characterization. Mater Res Bull 33(10):1457–1465. doi:10.1016/s0025-5408(98)00143-3
Blonska-Tabero A (2010) Pb2Fe2V4O15—A new phase forming in the system FeVO4–Pb2V2O7. J Alloy Compd 508(1):42–46. doi:10.1016/j.jallcom.2010.08.028
Blonska-Tabero A (2009) A new iron lead vanadate Pb2FeV3O11: synthesis and some properties. Mater Res Bull 44(8):1621–1625. doi:10.1016/j.materresbull.2009.04.015
Liu H, Hu C, Wang ZL (2006) Composite-hydroxide-mediated approach for the synthesis of nanostructures of complex functional-oxides. Nano Lett 6(7):1535–1540. doi:10.1021/nl061253e
Suárez-Gómez A, Saniger-Blesa JM, Calderón-Piñar F (2012) ‘Universal’ Synthesis of PZT (1−x)/x Submicrometric structures using highly stable colloidal dispersions: a bottom-up approach. In: Peláiz-Barranco A (ed) Advances in Ferroelectrics. InTech. doi:10.5772/51996
Suárez-Gómez A, Saniger-Blesa JM, Calderón-Piñar F (2010) A study on the stability of a PZT precursor solution based on the time evolution of mean particles size and pH. Mater Chem Phys 123(1):304–308. doi:10.1016/j.matchemphys.2010.04.017
Tolentino HCN, Ramos AY, Alves MCM, Barrea RA, Tamura E, Cezar JC, Watanabe N (2001) A 2.3 to 25 keV XAS beamline at LNLS. J Synchrotron Radiat 8(3):1040–1046. doi:10.1107/s0909049501005143
Suárez-Gómez A, Saniger-Blesa JM, Calderón-Piñar F (2011) A crystallization study of nanocrystalline PZT 53/47 granular arrays using a sol-gel based precursor. J Mater Sci Technol 27(6):489–496. doi:10.1016/s1005-0302(11)60096-0
Zyryanov VV, Lapina OB (2001) Mechanochemical synthesis and structure of new phases in the Pb–V–O system. Inorg Mater 37(3):264–270. doi:10.1023/a:1004169431601
Dimitrov V, Dimitriev Y (1990) Structure of glasses in PbO-V2O5 system. J Non-Cryst Solids 122(2):133–138. doi:10.1016/0022-3093(90)91058-y
Fotiev AA, Slobodin BV, Khodos MY (1988) Vanadaty: sostav, sintez, struktura, svoistva (Vanadates: Composition, Synthesis, Structure, Properties). Nauka, Moskva
Kawahara A (1967) La structure cristalline de la chervetite. Bulletin de la Societe Francaise de Mineralogie et de Cristallographie 90:279–284
Shannon RD, Calvo C (1973) Refinement of the crystal structure of synthetic chervetite, Pb2V2O7. Can J Chem 51(1):70–76. doi:10.1139/v73-010
Martin K, McCarthy G (1993) ICDD Grant-in-Aid. Card 47-1735. ICDD, North Dakota State University, Fargo, USA
Midorikawa M, Kashida H, Sawada A, Ishibashi Y (1980) Ferroelectricity in Pb3(VO4)2 crystal. J Phys Soc Jpn 49(3):1095–1097. doi:10.1143/jpsj.49.1095
Salje E, Iishi K (1977) Ferroelastic phase transitions in lead phosphate–vanadate Pb3(PxV1−xO4)2. AcCrA 33(3):399–408. doi:10.1107/s0567739477001065
Baran EJ, Pedregosa JC, Aymonino PJ (1975) Das Schwingungsspektrum von Pb2V2O7. Monatsh Chem 106(5):1085–1090. doi:10.1007/bf00906220
Weinstock N, Schulze H, Müller A (1973) Assignment of ν2 (E) and ν4 (F2) of tetrahedral species by the calculation of the relative Raman intensities: the vibrational spectra of VO4 3−, CrO4 2−, MoO4 2−, WO4 2−, MnO4 −, TcO4 −, ReO4 −, RuO4, and OsO4. J Chem Phys 59(9):5063. doi:10.1063/1.1680724
Baran EJ (1978) A correlation between the V—O—V bridge stretching frequencies and angle in divanadates. J Mol Struct 48(3):441–443. doi:10.1016/0022-2860(78)87254-8
Wing RM, Callahan KP (1969) Characterization of metal-oxygen bridge systems. Inorg Chem 8(4):871–874. doi:10.1021/ic50074a034
Brown RG, Ross SD (1972) The vibrational spectra of some condensed tetrahedral anions [X2O7]n−. Spectrochim Acta Part A 28(7):1263–1274. doi:10.1016/0584-8539(72)80096-5
Hezel A, Ross SD (1967) The vibrational spectra of some divalent metal pyrophosphates. Spectrochim Acta Part A 23(5):1583–1589. doi:10.1016/0584-8539(67)80381-7
Wong J, Messmer RP, Maylotte DH (1984) K-edge absorption spectra of selected vanadium compounds. Phys Rev B 30(10):5596–5610. doi:10.1103/PhysRevB.30.5596
Acknowledgments
This work has been supported by Project No. 221541/CUValles(DECyT)/P3e-2014, Project PROINPEP-2014/CUValles and Project PROMEP-NPTC No. UDG-PTC-1080. The authors would also like to thank to CNPEM, in particular to the LNNano and LNLS staff and, most of all, to Dr. C.A. Ospina Ramírez for his kind support with SEM characterizations. Besides, the help provided by Dr. C. Velásquez-Ordoñez, CUVALLES-UdG must also be acknowledged and highly appreciated. We also thank Dr. M. Saleta from Unicamp for help on XAFS acquisition.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suárez-Gómez, A., Figueroa, S.J.A., Lamas, D.G. et al. A crystallization and structural study of the compound Pb2V2O7 synthesized by a facile sol–gel-based chemical route. J Sol-Gel Sci Technol 75, 291–297 (2015). https://doi.org/10.1007/s10971-015-3698-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3698-0