Abstract
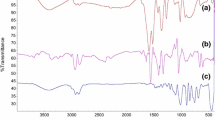
A synthetic exfoliated nanoclay smectite type, Laponite® S482, was incorporated as a functionalized load in a silica hybrid matrix synthesized by the sol–gel route. The previous functionalization was carried out through a “grafting” reaction with (3-glycidoxypropyl)trimethoxysilane (GPTMS) assisted by ultrasonic dispersion. The precursor sols were synthesized by acid-catalyzed hydrolytic condensation between tetraethoxysilane (TEOS) and functionalized GPTMS, a silver ions source was added in order to obtain a coating material with controlled silver releasing properties. Coatings were obtained by “dip-coating” on different substrates. Structural characterization of the coatings was conducted by SAXS and SEM-EDS, the results revealed a complex silica matrix with intercalated nanoclays, an organic fraction and a homogeneous content of Ag+. The electrochemical characterization was realized by EIS tests on stainless steel coated substrates AISI 316L type; the results showed good barriers properties and a high integrity of the coatings loaded with nanoclay. The evolution of the release of Ag+ ions was studied by XRF, through exposing the coatings to a leaching process at steady state and determining the residual content of Ag within the coat at different immersion times. It was found that the addition of 1.5 wt. % of clay, in respect to condensed silica, decreased the initial diffusion rate of Ag+ ions at near the half part, allowing its potential use in the development of antibacterial coatings with longer terms of life.










Similar content being viewed by others
References
Schmidt H, Jonschker G, Goedicke S, Mennig M (2000) The Sol–gel process as a basic technology for nanoparticle-dispersed inorganic-organic composites. J Sol-Gel Sci Technol 19:39–51. https://doi.org/10.1023/A:1008706003996
Pandey S, Mishra SB (2011) Sol-gel derived organic-inorganic hybrid materials: synthesis, characterizations and applications. J Sol-Gel Sci Technol 59:73–94. https://doi.org/10.1007/s10971-011-2465-0
Olivier MG, Fedel M, Sciamanna V et al. (2011) Study of the effect of nanoclay incorporation on the rheological properties and corrosion protection by a silane layer. Prog Org Coat 72:15–20. https://doi.org/10.1016/j.porgcoat.2010.11.022
Yeh J-M, Chen C-L, Chen Y-C et al. (2002) Enhancement of corrosion protection effect of poly(o-ethoxyaniline) via the formation of poly(o-ethoxyaniline)–clay nanocomposite materials. Polym 43:2729–2736. https://doi.org/10.1016/S0032-3861(02)00005-8
Herrera Alonso R, Estevez L, Lian H et al. (2009) Nafion-clay nanocomposite membranes: morphology and properties. Polym 50:2402–2410. https://doi.org/10.1016/j.polymer.2009.03.020
Deflorian F, Rossi S, Fedel M, Motte C (2010) Electrochemical investigation of high-performance silane sol–gel films containing clay nanoparticles. Prog Org Coat 69:158–166. https://doi.org/10.1016/j.porgcoat.2010.04.007
Seeni Meera KM, Murali Sankar R, Murali A et al. (2012) Sol-gel network silica/modified montmorillonite clay hybrid nanocomposites for hydrophobic surface coatings. Colloids Surf B Biointerfaces 90:204–210. https://doi.org/10.1016/j.colsurfb.2011.10.018
Joncoux-Chabrol K, Bonino JP, Gressier M et al. (2012) Improvement of barrier properties of a hybrid sol–gel coating by incorporation of synthetic talc-like phyllosilicates for corrosion protection of a carbon steel. Surf Coat Technol 206:2884–2891. https://doi.org/10.1016/j.surfcoat.2011.12.017
Santana I, Pepe A, Schreiner W, et al (2015) Hybrid sol–gel coatings containing clay nanoparticles for corrosion protection of mild steel. Electrochim Acta. https://doi.org/10.1016/j.electacta.2016.01.214
Jones SA, Bowler PG, Walker M, Parsons D (2004) Controlling wound bioburden with a novel silver-containing Hydrofiber?? dressing. Wound Repair Regen 12:288–294. https://doi.org/10.1111/j.1067-1927.2004.012304.x
Silver S, Phung LT (1996) Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol 50:753–789. https://doi.org/10.1146/annurev.micro.50.1.753
Crabtree JH, Burchette RJ, Siddiqi RA et al. (2003) The efficacy of silver-ion implanted catheters in reducing peritoneal dialysis-related infections. Perit Dial Int 23:368–374
Zhao G, Stevens SE (1998) Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. BioMetals 11:27–32. https://doi.org/10.1023/A:1009253223055
Ando Y, Miyamoto H, Noda I et al. (2010) Calcium phosphate coating containing silver shows high antibacterial activity and low cytotoxicity and inhibits bacterial adhesion. Mater Sci Eng C 30:175–180. https://doi.org/10.1016/j.msec.2009.09.015
Sun B, Sun SQ, Li T, Zhang WQ (2007) Preparation and antibacterial activities of Ag-doped SiO 2-TiO2 composite films by liquid phase deposition (LPD) method. J Mater Sci 42:10085–10089. https://doi.org/10.1007/s10853-007-2109-5
Jeanmonod P, Laschke MW, Gola N et al. (2014) Early host tissue response to different types of vascular prostheses coated with silver acetate or vaporized metallic silver. Eur J Vasc Endovasc Surg 47:680–688. https://doi.org/10.1016/j.ejvs.2014.03.006
Ferreri I, Lopes V, Calderon VS et al. (2014) Study of the effect of the silver content on the structural and mechanical behavior of Ag-ZrCN coatings for orthopedic prostheses. Mater Sci Eng C 42:782–790. https://doi.org/10.1016/j.msec.2014.06.007
Qin H, Cao H, Zhao Y et al. (2014) In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials 35:9114–9125. https://doi.org/10.1016/j.biomaterials.2014.07.040
Abboud EC, Settle JC, Legare TB et al. (2014) Silver-based dressings for the reduction of surgical site infection: Review of current experience and recommendation for future studies. Burns 40:S30–S39. https://doi.org/10.1016/j.burns.2014.09.011
Dal Lago V, França de Oliveira L, de Almeida, Gonçalves K et al. (2011) Size-selective silver nanoparticles: future of biomedical devices with enhanced bactericidal properties. J Mater Chem 21:12267. https://doi.org/10.1039/c1jm12297e
Orazem ME, Tribollet B (2008) Electrochemical Impedance Spectroscopy. Analysis. https://doi.org/10.1002/9780470381588
Mcintyre JM, Pham HQ (1996) Electrochemical impedance spectroscopy; coatings optimizations a tool for organic. Prog Org Coat 27:201–207. https://doi.org/10.1016/0300-9440(95)00532-3
Huang TC, Toraya H, Blanton TN, Wu Y (1993) X-ray powder diffraction analysis of silver behenate, a possible low-angle diffraction standard. J Appl Crystallogr 3:180–184. https://doi.org/10.1107/S0021889892009762
Hammersley AP (2016) FIT2D: a multi-purpose data reduction, analysis and visualization program. J Appl Crystallogr 49:646–652. https://doi.org/10.1107/S1600576716000455
Teubner M, Strey R (1987) Origin of the scattering peak in microemulsions. J Chem Phys 87:3195–3200. https://doi.org/10.1063/1.453006
Laity PR, Taylor JE, Wong SS et al. (2004) A review of small-angle scattering models for random segmented poly(ether-urethane) copolymers. Polym 45:7273–7291. https://doi.org/10.1016/j.polymer.2004.08.033
Akhavan O (2009) Silver nanocube crystals on titanium nitride buffer layer. J Phys D Appl Phys. https://doi.org/10.1088/0022-3727/42/10/105305
Fang J, Leufke PM, Kruk R et al. (2010) External electric field driven 3D ordering architecture of silver (I) oxide meso-superstructures. Nano Today 5:175–182. https://doi.org/10.1016/j.nantod.2010.05.002
Dhoondia ZH, Chakraborty H (2012) Lactobacillus mediated synthesis of silver oxide nanoparticles. Nanomater Nanotechnol 2:15. https://doi.org/10.5772/55741
Zheng S, Li J (2010) Inorganic-organic sol–gel hybrid coatings for corrosion protection of metals. J Sol-Gel Sci Technol 54:174–187. https://doi.org/10.1007/s10971-010-2173-1
Balgude D, Sabnis A (2012) Sol-gel derived hybrid coatings as an environment friendly surface treatment for corrosion protection of metals and their alloys. J Sol-Gel Sci Technol 64:124–134. https://doi.org/10.1007/s10971-012-2838-z
Carmezim MJ, Simões AM, Montemor MF, Da Cunha Belo M (2005) Capacitance behaviour of passive films on ferritic and austenitic stainless steel. Corros Sci 47:581–591
Yasakau KA, Zheludkevich ML, Karavai OV, Ferreira MGS (2008) Influence of inhibitor addition on the corrosion protection performance of sol-gel coatings on AA2024. Prog Org Coat 63:352–361. https://doi.org/10.1016/j.porgcoat.2007.12.002
Liu C, Bi Q, Leyland A, Matthews A (2003) An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part II.: EIS interpretation of corrosion behaviour. Corros Sci 45:1257–1273. https://doi.org/10.1016/S0010-938X(02)00214-7
Mansfeld F (1995) Use of electrochemical impedance spectroscopy for the study of corrosion protection by polymer coatings I—I I. J Appl Electrochem 25:187–202. https://doi.org/10.1007/BF00262955
Akhavan O (2009) Lasting antibacterial activities of Ag-TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation. J Colloid Interface Sci 336:117–124. https://doi.org/10.1016/j.jcis.2009.03.018
Akhavan O, Ghaderi E (2009) Bactericidal effects of Ag nanoparticles immobilized on surface of SiO2 thin film with high concentration. Curr Appl Phys 9:1381–1385. https://doi.org/10.1016/j.cap.2009.03.003
Liu Y, Wang X, Yang F, Yang X (2008) Excellent antimicrobial properties of mesoporous anatase TiO2 and Ag/TiO2 composite films. Microporous Mesoporous Mater 114:431–439. https://doi.org/10.1016/j.micromeso.2008.01.032
Kawashita M, Toda S, Kim H-M et al. (2003) Preparation of antibacterial silver-doped silica glass microspheres. J Biomed Mater Res 66A:266–274. https://doi.org/10.1002/jbm.a.10547
Akhavan O, Abdolahad M, Abdi Y, Mohajerzadeh S (2011) Silver nanoparticles within vertically aligned multi-wall carbon nanotubes with open tips for antibacterial purposes. J Mater Chem 21:387–393. https://doi.org/10.1039/C0JM02395G
Akhavan O, Ghaderi E (2009) Capping antibacterial Ag nanorods aligned on Ti interlayer by mesoporous TiO2 layer. Surf Coat Technol 203:3123–3128. https://doi.org/10.1016/j.surfcoat.2009.03.033
Mahltig B, Fiedler D, Böttcher H (2004) Antimicrobial Sol – Gel Coatings. J Sol-Gel Sci Technol 32:219–222
Stobie N, Duffy B, McCormack DE et al. (2008) Prevention of Staphylococcus epidermidis biofilm formation using a low-temperature processed silver-doped phenyltriethoxysilane sol-gel coating. Biomaterials 29:963–969. https://doi.org/10.1016/j.biomaterials.2007.10.057
Procaccini RA, Studdert CA, Pellice SA (2014) Silver doped silica-methyl hybrid coatings. Structural evolution and antibacterial properties. Surf Coat Technol 244:92–97. https://doi.org/10.1016/j.surfcoat.2014.01.036
Procaccini R, Bouchet A, Pastore JI et al. (2016) Silver-functionalized methyl-silica hybrid materials as antibacterial coatings on surgical-grade stainless steel. Prog Org Coat 97:28–36. https://doi.org/10.1016/j.porgcoat.2016.03.012
Acknowledgements
Authors want to acknowledge the Argentine National Council of Scientific and Technical Researches (CONICET, PIP 2012-0434) and the National Synchrotron Light Laboratory of Brazil (LNLS, Project 6780/10, proposal D11A-SAXS1-15291) for the financial supports. In addition, Mr. Martín E. Lere is gratefully acknowledged for his helpful technical collaboration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
-
Successful incorporation of exfoliated clay nanoparticles in silver-rich hybrid coatings.
-
Obtained hybrid matrices present a spinodal-like bi-continuous structure highly favorable to the Ag+ ions mobility.
-
Incorporation of low amounts of Laponite® S482 limited the superficial agglomeration of silver in the coatings.
-
The addition of clay nanoparticles had a diffusional-control effect on the mobility of Ag+ ions within the hybrid materials.
-
Low concentrations of Laponite® S482 improved the silver releasing behavior and extended the lifespan of the material, allowing a potential antibacterial application.
Rights and permissions
About this article
Cite this article
Giraldo Mejía, H.F., Procaccini, R.A. & Pellice, S.A. Synthesis and characterization of silver-rich coatings loaded with functionalized clay nanoparticles. J Sol-Gel Sci Technol 85, 529–538 (2018). https://doi.org/10.1007/s10971-018-4600-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4600-7