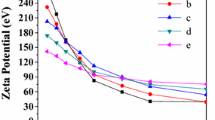
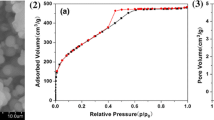
Abstract
Silicon dioxide (SiO2) obtained by Sol–Gel methods is widely used as adsorbents, catalytic supports, filter membranes and in drugs delivery, among others. For most of the applications, surface area and porosity are key parameters that should be controlled, depending on the purpose of the material. These characteristics depend on the chemistry of the precursors in solution. Silicon alkoxides are commonly used as precursors, where the chemical pathway to produce sols and then gels depends on several factors such as water/alcohol ratio, pH, type of catalyst, temperature, etc. In order to control the microstructural characteristics of SiO2, it is necessary to understand the effect of the different chemical components on the hydrolysis-condensation reactions. In this work, we explored the acid-catalyzed hydrolysis and condensation reactions of silicon tetra-ethyl-alkoxide (TEOS) employing three common acids: HF, HCl, and HNO3. Gel formation kinetics was studied by low field nuclear magnetic resonance. Structural evolution of gels and xerogels at the nanoscale was determined by small angle X-ray scattering (SAXS). The microstructure of xerogels was determined by nitrogen adsorption (BET method), and by scanning and transmission electron microscopy (SEM and TEM, respectively). The final SiO2 products revealed different porosity type and texture depending on the acid employed, which are related to the chemical pathway during the sol–gel transition.

Highlights
-
SiO2 gelation time determined by LF-1H-NMR decreases with nucleophilicity of acid catalyst anion.
-
SiO2 gels microstructure change slightly with aging for HF catalyst but quite remarkably with HNO3/HCl.
-
SiO2 xerogel microstructure is defined during drying for HF catalyst but during aging for HNO3/HCl.
-
HF as catalyst lead to mesoporous xerogels, while HNO3/HCl lead to microporous ones.
-
Catalyst selection allows tailoring SiO2 morphologies and microstructure for different applications.






Similar content being viewed by others
References
Li F, Huang X, Liu J-X, Zhang G-J (2020) Sol-gel derived porous ultra-high temperature ceramics. J Adv Ceram 9:1–16. https://doi.org/10.1007/s40145-019-0332-6
Baino F, Fiorilli S, Vitale-Brovarone C (2017) Composite biomaterials based on sol-gel mesoporous silicate glasses: a review. Bioengineering 4:15. https://doi.org/10.3390/bioengineering4010015
Ishikawa K, Garskaite E, Kareiva A (2020) Sol–gel synthesis of calcium phosphate-based biomaterials—a review of environmentally benign, simple, and effective synthesis routes. J Sol-Gel Sci Technol 94:551–572. https://doi.org/10.1007/s10971-020-05245-8
Parashar M, Shukla VK, Singh R (2020) Metal oxides nanoparticles via sol–gel method: a review on synthesis, characterization and applications. J Mater Sci Mater Electron 31:3729–3749. https://doi.org/10.1007/s10854-020-02994-8
Vera ML, Cánneva A, Huck-Iriart C, Requejo FG, Gonzalez MC, Dell’Arciprete ML, Calvo A (2017) Fluorescent silica nanoparticles with chemically reactive surface: Controlling spatial distribution in one-step synthesis. J Colloid Interface Sci 496:456–464. https://doi.org/10.1016/j.jcis.2017.02.040
Marzouk SS, Naddeo V, Banat F, Hasan SW (2021) Preparation of TiO2/SiO2 ceramic membranes via dip coating for the treatment of produced water. Chemosphere 273:129684. https://doi.org/10.1016/j.chemosphere.2021.129684
de Lima Almeida L, da Silva Ferreira DWF, de Andrade Santana J, Huck-Iriart C, Kunst SR, Zoppas Ferreira J, Trindade Oliveira C, Vitorino Sarmento VH (2020) Effect of the addition of calcium salts on the structure and anticorrosion properties of siloxane-poly(hydroxyethyl methacrylate) hybrid coating applied on Ti-6Al-4V alloy. J Sol-Gel Sci Technol 96:690–701. https://doi.org/10.1007/s10971-020-05394-w
Dehghanghadikolaei A, Ansary J, Ghoreishi R (2018) Sol-gel process applications: A mini-review. Proc Nat Res Soc 2:02008. https://doi.org/10.11605/j.pnrs.201802008
Brinker CJ, Scherer GW (1990) Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. Academic Press, San Diego, CA, USA
Livage J (1986) Synthesis, Structure and Applications of TiO2 Gels. MRS Proc 72:323. https://doi.org/10.1557/PROC-72-323
Babonneau F, Diré S, Bonhomme-Coury L, Livage J (1994) Sol—Gel Synthesis of Heterometallic Oxopolymers. In: Inorganic and Organometallic Polymers II, pp134-148. American Chemical Society, Washington DC, USA
Siouffi A-M (2003) Silica gel-based monoliths prepared by the sol–gel method: facts and figures. J Chromatogr A 1000:801–818. https://doi.org/10.1016/S0021-9673(03)00510-7
Jafarzadeh M, Rahman IA, Sipaut CS (2009) Synthesis of silica nanoparticles by modified sol–gel process: the effect of mixing modes of the reactants and drying techniques. J Sol-Gel Sci Technol 50:328–336. https://doi.org/10.1007/s10971-009-1958-6
Rodrigues Mota TL, Marques de Oliveira AP, Nunes EHM, Houmard M (2017) Simple process for preparing mesoporous sol-gel silica adsorbents with high water adsorption capacities. Microporous Mesoporous Mater 253:177–182. https://doi.org/10.1016/j.micromeso.2017.07.010
Rodrigues Mota TL, Moura Gomes AL, Guimarães Palhares H, Martins Nunes EH, Houmard M (2019) Influence of the synthesis parameters on the mesoporous structure and adsorption behavior of silica xerogels fabricated by sol–gel technique. J Sol-Gel Sci Technol 92:681–694. https://doi.org/10.1007/s10971-019-05131-y
Kato M, Sakai‐Kato K, Toyo’oka T (2005) Silica sol-gel monolithic materials and their use in a variety of applications. J Sep Sci 28:1893–1908. https://doi.org/10.1002/jssc.200500225
Farjood M, Zanjanchi MA (2020) A new synthesis methodology for SiO2 gel-based nanostructures and their application for elimination of dye pollutants. New J Chem 44:5386–5395. https://doi.org/10.1039/D0NJ00093K
Deshmukh K, Kovářík T, Křenek T, Stich T, Docheva D (2020) Microstructural evaluation and thermal properties of sol-gel derived silica-titania based porous glasses. J Phys Conf Ser 1527:012031. https://doi.org/10.1088/1742-6596/1527/1/012031
Gonçalves MC (2018) Sol-gel silica nanoparticles in medicine: a natural choice. Design, synthesis and products. Molecules 23:2021. https://doi.org/10.3390/molecules23082021
Lei Q, Guo J, Noureddine A, Wang A, Wuttke S, Brinker CJ, Zhu W (2020) Sol–gel‐based advanced porous silica materials for biomedical applications. Adv Funct Mater 30:1909539. https://doi.org/10.1002/adfm.201909539
Guzel Kaya G, Deveci H (2020) Synergistic effects of silica aerogels/xerogels on properties of polymer composites: a review. J Ind Eng Chem 89:13–27. https://doi.org/10.1016/j.jiec.2020.05.019
de Souza T, da CC, Brasil A, Ladeira LO, Houmard M (2019) Influence of the nanostructure of silica supports on the growth and morphology of MWCNTs synthesized by CCVD method. Ceram Int 45:13297–13307. https://doi.org/10.1016/j.ceramint.2019.04.019
Framery E, Mutin PH (2002) 29Si MAS-NMR study of silica gels and xerogels: influence of the catalyst. J Sol-Gel Sci Technol 24:191–195. https://doi.org/10.1023/A:1015391922572
Dumas RL, Tejedor-Tejedor I, Anderson MA (1998) Dependence of SiO2> gel structure on gelation conditions and sol reaction temperature as followed by FTIR and nitrogen adsorption measurements. J Porous Mater 5:95–101. https://doi.org/10.1023/A:1009682117946
Chou KT, Lee BI (1993) Properties of silica gels prepared from high-acid hydrolysis of tetraethoxysilane. Ceram Int 19:315–325. https://doi.org/10.1016/0272-8842(93)90044-R
Meixner DL, Dyer PN (1999) Influence of sol-gel synthesis parameters on the microstructure of particulate silica xerogels. J Sol-Gel Sci Technol 14:223–232. https://doi.org/10.1023/A:1008774827602
Cihlář J (1993) Hydrolysis and polycondensation of ethyl silicates. 1. Effect of pH and catalyst on the hydrolysis and polycondensation of tetraethoxysilane (TEOS). Colloids Surf Physicochem Eng Asp 70:239–251. https://doi.org/10.1016/0927-7757(93)80298-S
Pope EJA, Mackenzie JD (1986) Sol-gel processing of silica: II. The role of the catalyst. J Non-Cryst Solids 87:185–198. https://doi.org/10.1016/S0022-3093(86)80078-3
Chen F, Chen C, Zhao D et al. (2020) On-line monitoring of the sol-gel transition temperature of thermosensitive chitosan/β-glycerophosphate hydrogels by low field NMR. Carbohydr Polym 238:116196. https://doi.org/10.1016/j.carbpol.2020.116196
Barbosa-Cánovas GV, Jr AJF, Schmidt SJ, Labuza TP (2020) Water Activity in Foods: Fundamentals and Applications. John Wiley & Sons; Hoboken NJ, USA.
Silletta EV, Velasco MI, Gómez CG, Acosta RH, Strumia MC, Monti GA (2014) Evaporation Kinetics in Swollen Porous Polymeric Networks. Langmuir 30:4129–4136. https://doi.org/10.1021/la500031t
Ok S, Hwang B, Liu T, Welch S, Sheets JM, Cole DR, Liu KH, Mou CY (2020) Fluid Behavior in Nanoporous Silica. Frontiers in Chemistry 8: 734–754. https://doi.org/10.3389/fchem.2020.00734
Wei M, Zhang L, Xiong Y, Li J, Peng P (2016) Nanopore structure characterization for organic-rich shale using the non-local-density functional theory by a combination of N2 and CO2 adsorption. Microporous Mesoporous Mater 227:88–94. https://doi.org/10.1016/j.micromeso.2016.02.050
Ravikovitch PI, Haller GL, Neimark AV (1998) Density functional theory model for calculating pore size distributions: pore structure of nanoporous catalysts. Adv Colloid Interface Sci 76–77:203–226. https://doi.org/10.1016/S0001-8686(98)00047-5
Carr HY, Purcell EM (1954) Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys Rev 94:630–638. https://doi.org/10.1103/PhysRev.94.630
Meiboom S, Gill D (1958) Modified spin‐echo method for measuring nuclear relaxation times. Rev Sci Instrum 29:688–691. https://doi.org/10.1063/1.1716296
Sánchez-Alonso I, Moreno P, Careche M (2014) Low field nuclear magnetic resonance (LF-NMR) relaxometry in hake (Merluccius merluccius, L.) muscle after different freezing and storage conditions. Food Chem 153:250–257. https://doi.org/10.1016/j.foodchem.2013.12.060
Borgia GC, Brown RJS, Fantazzini P (1998) Uniform-Penalty Inversion of Multiexponential Decay Data. J Magn Reson 132:65–77. https://doi.org/10.1006/jmre.1998.1387
Hammouda B (2010) A new Guinier–Porod model. J Appl Crystallogr 43:716–719. https://doi.org/10.1107/S0021889810015773
Teixeira J (1988) Small-angle scattering by fractal systems. J Appl Crystallogr 21:781–785. https://doi.org/10.1107/S0021889888000263
Craievich AF (2016) Small-Angle X-ray Scattering by Nanostructured Materials. In: Klein L, Aparicio M, Jitianu A (eds) Handbook of Sol-Gel Science and Technology, pp 1-46. Springer International Publishing, Cham, Denmark
Newville M, Stensitzki T, Allen DB, Ingargiola A (2014) LMFIT: Non-Linear Least-Square Minimization and Curve-Fitting for Python. Zenodo. https://zenodo.org/badge/DOI/10.5281/zenodo.11813.svg
Lv C, Tian H, Zhang X, Xiang A (2018) LF-NMR analysis of the water mobility, state and distribution in sorbitol plasticized polyvinyl alcohol films. Polym Test 70:67–72. https://doi.org/10.1016/j.polymertesting.2018.06.024
Li Y, Li X, Chen C, Zhao D, Su Z, Ma G, Yu R (2017) Sol–gel transition characterization of thermosensitive hydrogels based on water mobility variation provided by low field NMR. J Polym Res 24:25. https://doi.org/10.1007/s10965-017-1185-8
Brinker CJ, Tallant DR, Roth EP, Ashley CS (1986) Sol-gel transition in simple silicates: III. Structural studies during densification. J Non-Cryst Solids 82:117–126. https://doi.org/10.1016/0022-3093(86)90119-5
de Lange RSA, Hekkink JHA, Keizer K, Burggraaf AJ (1995) Polymeric-silica-based sols for membrane modification applications: sol-gel synthesis and characterization with SAXS. J Non-Cryst Solids 191:1–16. https://doi.org/10.1016/0022-3093(95)00291-X
Londoño OM, Tancredi P, Rivas P, Muraca D, Socolovsky LM, Knobel M (2018) Small-Angle X-Ray Scattering to Analyze the Morphological Properties of Nanoparticulated Systems. In: Sharma SK (ed) Handbook of Materials Characterization. Springer International Publishing, Cham, Danmark, pp 37–75
Nair BN, Elferink WJ, Keizer K, Verweij H (1996) Sol–Gel Synthesis and Characterization of Microporous Silica Membranes I: SAXS Study on the Growth of Polymeric Structures. J Colloid Interface Sci 178:565–570. https://doi.org/10.1006/jcis.1996.0152
Sing, KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquérol J, Siemieniewska T (1985). Reporting physisorption data for gas/solid systems. Pure & Appl. Chem. 57(4):603–619
Corriu RJP, Leclercq D, Lefèvre P, Mutin PH, Vioux A (1992) Preparation of monolithic gels from silicon halides by a non-hydrolytic sol-gel process. J Non-Cryst Solids 146:301–303. https://doi.org/10.1016/S0022-3093(05)80505-8
Ro JC, Chung IJ (1989) Sol-gel kinetics of tetraethylorthosilicate (TEOS) in acid catalyst. J Non-Cryst Solids 110:26–32. https://doi.org/10.1016/0022-3093(89)90178-6
Ro JC, In JC (1991) Structures and properties of silica gels prepared by the sol—gel method. Journal of Non-Crystalline Solids 130(1):8–17. https://doi.org/10.1016/0022-3093(91)90151-u
Morales NJ, Goyanes S, Chiliotte C, Bekeris V, Candal RJ, Rubiolo GH (2013) One-step chemical vapor deposition synthesis of magnetic CNT–hercynite (FeAl2O4) hybrids with good aqueous colloidal stability. Carbon 61:515–524. https://doi.org/10.1016/j.carbon.2013.04.106
Erdem İ (2017) Sol-gel applications for ceramic membrane preparation. AIP Conference Proceedings 1809, 020011. https://doi.org/10.1063/1.4975426
Funding
This work was supported through the University of Buenos Aires (project UBACyT 20020090100297), through the National Agency for the Promotion of Science and Technology (ANPCyT, projects PICT 2016-2940 and PICT 2017-3150). The authors wish to thank the National Laboratory of Synchrotron Radiation (LNLS) for the support through project SAXS1 11755. Cristián Huck-Iriart, María Lidia Herrera and Roberto Jorge Candal are members of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Infomation
Rights and permissions
About this article
Cite this article
Huck-Iriart, C., Morales, N.J., Herrera, M.L. et al. Micro to mesoporous SiO2xerogels: the effect of acid catalyst type in sol–gel process. J Sol-Gel Sci Technol 102, 197–207 (2022). https://doi.org/10.1007/s10971-021-05601-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05601-2