Abstract
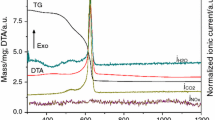
A porous nickel–8 mol% yttria stabilized zirconia (Ni–8YSZ) composite, used as anode for solid oxide fuel cell, was obtained by reduction of NiO–8YSZ cermet. The first goal was the evaluation of the temperature effect of powder processing by thermogravimetry. In addition, the influence of porosity in the reduction kinetic of the sample sintered at 1450 °C was evaluated. The final porosity produced in NiO–8YSZ composite by pore former was 30.4 and 37.9 vol.%, respectively, for 10 and 15 mass% of corn starch. The sample with 15 mass% of corn starch promotes a reduction rate almost twice higher than sample with 10 mass% of corn starch. The porosity introduced by the reduction of NiO was 23 vol.%.







Similar content being viewed by others
References
Dikwal CM, Bujalski W, Kendall K. The effect of temperature gradients on thermal cycling and isothermal ageing of micro-tubular solid oxide fuel cells. J Power Sources. 2008;193:241–8.
Kawada T, Sakai N, Yokokawa H, Dokiya M, Mori M, Iwata T. Characteristics of slurry-coated nickel zirconia cermet anodes for solid oxide fuel cells. Solid State Ionics. 1990;137:3042–7.
Bieberle A, Méier LP, Gauckler LJ. The electrochemistry of Ni pattern anodes used as solid oxide fuel cell model electrodes. J Electrochem Soc. 2001;148:646–56.
Lee JH, Moon H, Lee HW, Kim J, Kim JD, Yoon KH. Quantitative analysis of microstructure and its related electrical property of SOFC anode, Ni–YSZ cermet. Solid State Ionics. 2002;148:15–26.
Rojas AR, Esparaza-Ponce HE, Fuents L. In situ X-ray Rietveld analysis of Ni–YSZ solid oxide fuel cell anodes during NiO reduction in H2. J Phys D: Appl Phys. 2005;38:2276–82.
Borer AL, Bronnimann C, Prins R. ZrO2-promoted Rh/SiO2 catalysts in CO hydrogenation and temperature-programmed reduction. J Catal. 1994;145:516–25.
Guglielminotti E, Giamello E, Pinna F, Strukul G, Martinengo S, Zanderighi L. Elementary steps in CO hydrogenation on Rh catalysts supported on ZrO2 and Mo/ZrO2. J Catal. 1994;146:422–36.
Dow WP, Wang YP, Huang TJ. Yttria-stabilized zirconia supported copper oxide catalyst I. Effect of oxygen vacancy of support on copper oxide. J Catal. 1996;160:155–70.
Haslam JJ, Pham AQ, Chung BW, DiCarlo JF, Glass RS. Effects of the use of pore formers on performance of an anode supported solid oxide fuel cell. J Am Ceram Soc. 2005;88:513–8.
Tietz F, Dias FJ, Simwonis D, Stover D. Evaluation of commercial nickel oxide powders for components in solid oxide fuel cells. J Eur Ceram Soc. 2000;20:1023–34.
Grgicak CM, Green RG, Du WF, Giorgi JB. SOFC anodes for direct oxidation of hydrogen and methane fuels containing H2S. J Am Ceram Soc. 2005;88:3081–7.
Marinsek M, Zupan K, Maeek J. Ni–YSZ cermet anodes prepared by citrate/nitrate combustion synthesis. J Power Sources. 2002;106:178–88.
Guo SLR, Li J, Chen Y, Liu W. Synthesis of NiO–ZrO2 powders for solid oxide fuel cells. Ceram Int. 2003;29:883–6.
Yoshito WK, Ussui V, Lazar DRR, Paschoal JOA. Synthesis and characterization of NiO–8YSZ powders by co-precipitation route. Mater Sci Forum. 2005;498–499:612–7.
Ussui V, Leitão F, Yamagata C, Menezes CAB, Lazar DRR, Paschoal JOA. Synthesis of ZrO2-based ceramics for applications in SOFC. Mater Sci Forum. 2003;416–418:681–6.
ASTM C20-00. Standard test methods for apparent porosity, water absorption, apparent specific gravity, and bulk density of burned refractory brick and shape by boiling water; 2005.
Wang Y, Walter ME, Sabolsky K, Seabaugh MM. Effects of powder sizes and reduction parameters on the strength of Ni–YSZ anodes. Solid State Ionics. 2006;177:1517–27.
Sanson A, Pinasco P, Roncari E. Influence of pore formers on slurry composition and microstructure of tape cast supporting anodes for SOFCs. J Eur Ceram Soc. 2008;28:1221–6.
Gregorová E, Pabst W, Bohacenko I. Characterization of different starch types for their application in ceramic processing. J Eur Ceram Soc. 2006;26:1301–9.
Acknowledgements
The authors wish to thank to Celso Vieira de Morais, Nildemar Aparecido M. Ferreira, René de Oliveira for their assistance in the characterization of NiO–YSZ composite, CNPq, FAPESP and CAPES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshito, W.K., Matos, J.R., Ussui, V. et al. Reduction kinetics of NiO–YSZ composite for application in solid oxide fuel cell. J Therm Anal Calorim 97, 303–308 (2009). https://doi.org/10.1007/s10973-009-0237-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0237-7