Abstract
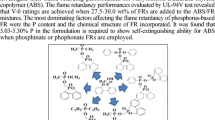
Organohalogen flame retardants, particularly brominated aromatics, are popular, effective, low cost, and widely used in the plastics industry. However, an increasing concern about persistence in the environment and potential negative health effects of these materials has generated intense interest in the development of alternatives. Ideally, these should have all the positive attributes of the materials that will be replaced. In addition, it is desirable that the new materials be as “green” as possible, e.g., based on renewable resources and be degradable to nontoxic products in the environment. A series of new, non-halogenated flame retardants based on tartaric acid is being developed. Tartaric acid is a by-product of the wine industry and is readily available locally on an annual basis (Michigan is the thirteenth largest producer of wine in the U.S.). It can be readily converted to the corresponding diethyl ester. This ester may serve as the base for the development of a series of new, non-halogenated flame-retarding agents. The presence of the reactive hydroxyl groups allows the introduction of a variety of phosphorus-containing moieties. For example, treatment of diethyl tartrate with diphenylphosphinyl chloride generates diethyl 2,3-di(diphenylphosphinato)-1,4-butanedioate. This material may serve as a monomer for the preparation of various phosphorus-containing polymers and oligomers via step-growth transesterification. The thermal stability of this compound has been assessed by thermogravimetry.









Similar content being viewed by others
References
Troitzsch J. Plastics flammability handbook: principles, regulations, testing and approval. 3rd ed. Cincinnati: Hanser Gardner Publications; 2004.
Georlette P, Simons J, Costa L. Halogen-containing fire-retardant compounds. In: Grand AF, Wilkie CW, editors. Fire retardancy of polymeric materials. New York: Marcel Dekker Inc.; 2000. pp. 245–84.
Gann RC. Flame retardants: overview. In: Kroschwitz J, Howe-Grant M, editors. Kirk-Othemer encyclopedia of chemical technology, vol. 10. New York: Wiley; 1993. pp. 930–936.
Green J. A review of phosphorus-containing flame retardants. J Fire Sci. 1992;10(6):470–87.
Weil ED. Phosphorus-based flame retardants. In: Engel RE, editor. Handbook of organophosphorus chemistry. New York: Marcel Dekker; 1992.
Weil ED, Levchik SV, Ravey M, Zhu WM. A survey of recent progress in phosphorus-based flame retardants and some mode of action studies. Phosphorus Sulfur Silicon Relat Elem. 1999;144–146:17–20.
Lu SY, Hamerton I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog Polym Sci. 2002;27(8):1661–712.
Davis J. The technology of halogen-free flame retardant additives for polymeric systems. Eng Plast. 1996;9(5):403–19.
Law RJ, Allchin CR, de Boer J, Covaci A, Herzke D, Lepom P, Morris S, Tronczynski J, de Wit CA. Levels and trends of brominated flame retardants in the European environment. Chemosphere. 2006;64:187–208.
de Wit C, Alace M, Muir D. Levels and trends of brominated flame retardants in the Arctic. Chemosphere. 2006;64:209–33.
Pepich BV, Prakash B, Domino MM, Dattilio TA. Development of U.S. EPA method 527 for the analysis of selected pesticides and flame retardants in the UCMR survey. Eviron Sci Technol 2005;39(13):4996–5004 (references cited therein).
Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112(1):9–17.
Alaee M, Wenning RJ. The significance of brominated flame retardants in the environment: current understanding, issues and challenges. Chemosphere. 2002;46:579–82.
Wheler EK, Hovander L, Bergmen A. New organohalogens in human plasma. Identification and quantification. Organohalog Compds. 1997;33:420–5.
Artner J, Ciesielski M, Walter O, Doring M, Perez RM, Sandler JKW, Altstadt V, Schartel B. A novel DOPO-based diamine as hardener and flame retardant for epoxy resin systems. Macromol Mater Eng. 2008;293(6):503–14.
Ciesielski M, Schafer A, Doring M. Novel efficient DOPO-based flame-retardants for PWB relevant epoxy resins with high glass transition temperatures. Polym Adv Technol. 2008;19(6):507–15.
Schartel B, Balabanovich AI, Braun U, Knoll U, Artner J, Ciesielski M, Doring M, Perez RM, Sandler JKW, Altstadt V, Hoffman T, Pospiech D. Pyrolysis of epoxy resins and fire behavior of epoxy resin composites flame-retarded with 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide additives. J Appl Polym Sci. 2007;104(4):2260–9.
Perez RM, Sandler JKW, Altstadt V, Hoffman T, Pospiech D, Artner J, Ciesielski M, Doring M, Balabanovich AI, Knoll U, Braun U, Schartel B. Novel phosphorus-containing hardeners with tailored chemical structures for epoxy resins: synthesis and cured resin properties. J Appl Polym Sci. 2007;105(5):2744–59.
Perez RM, Sandler JKW, Altstadt V, Hoffman T, Pospiech D, Artner J, Ciesielski M, Doring M, Balabanovich AI, Schartel B. Effective halogen-free flame retardancy for a monocomponent polyfunctional epoxy using an oligomeric organophosphorus compound. J Mater Sci. 2006;41(24):8347–51.
Perez RM, Sandler JKW, Altstadt V, Hoffman T, Pospiech D, Artner J, Ciesielski M, Doring M, Braun U, Knoll U, Schartel B. Effective halogen-free flame retardants for carbon fiber-reinforced epoxy composites. J Mater Sci. 2006;41(15):4981–4.
Perez RM, Sandler JKW, Altstadt V, Hoffman T, Pospiech D, Ciesielski M, Doring M. Effect of DOP-based compounds on fire retardancy, thermal stability, and mechanical properties of DGEBA cured with 4, 4′-DDS. J Mater Sci. 2006;41(2):341–53.
Amerine MA, Berg HW, Cruess WV. The technology of wine making. Westport: The Avi Publishing Company Inc.; 1972.
Austin C. The science of wine. London: University of London Press Ltd.; 1968.
Pinney T. A history of wine in America: from prohibition to the present. Berkeley: University of California Press; 2005.
Wine regions of the United States. http://www.wineforeveryone.com/wine_regions_united_states.html. Accessed 11 July 2009.
Michigan wine and grape council. http://www.michiganwines.com/page.php?menu=maps. Accessed 11 July 2009.
Rivas B, Torrado A, Moldes AB, Dominguez JM. Tartaric acid recovery from distilled lees and use of the residual solid as an economic nutrient for Lactobacillus. J Agric Food Chem. 2006;54(20):7904–11.
Versari A, Castellari M, Spinabelli U, Galassi S. Recovery of tartaric acid from industrial enological wastes. J Chem Technol Biotechnol. 2001;76(5):485–8.
Brown EM, Henriques VD. Vinification in California wineries. Ind Eng Chem. 1935;27:1235–40.
Marsh GL, Joslyn MA. Effect of temperature on the precipitation rate of cream of tartar from wine. Ind Eng Chem. 1935;27:1252–7.
Zhou X, Lin W-J, Ye J-L, Huang P-Q. A versatile approach to pyrrolidine azasugars and homoazasugars based on a highly diastereoselective reductive benzyloxymethylation of protected tartarimide. Tetrahedron. 2007;63(7):6346–57.
Lemieux RU, Howard J. The O-inside conformation of 1,3:2,4-di-O-methylene-l-threitol. Can J Chem. 1963;41:393–8.
Baolina TV, Groyvnova IB, Petrovskii PV, Matroscov EI, Groyunova EI, Nifantev EE. One-pot synthesis of N-diphenylphosphorylureas. Doklady Chem. 2006;409:129–32.
Tyssee DA, Bausher LP, Haake P. Displacement at phosphorus by a mechanism with A1 character. Acid-catalyzed hydrolysis of phosphinanilides. J Am Chem Soc. 1978;95(24):8066–72.
Dhawan BD, Redmore D. o-Hydroxyaryl diphosphonic acids. J Org Chem. 1984;49(21):4018–21.
Annakutty KS, Kishore K. Flame retardant polyphosphate esters. 2. Condensation polymers of bisphenol A with alkyl phosphorodichloridates: synthesis, characterization and thermal studies. Polymer. 1988;29(4):762–4.
Liu Y-L, Hsiue G-H, Chiu Y-S, Jeng R-J, Ma C. Synthesis and flame-retardant properties of phosphorus-containing polymers based on poly(4-hydroxystyrene). J Appl Polym Sci. 1996;59(10):1619–25.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Howell, B.A., Carter, K.E. Thermal stability of phosphinated diethyl tartrate. J Therm Anal Calorim 102, 493–498 (2010). https://doi.org/10.1007/s10973-010-0875-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0875-9