Abstract
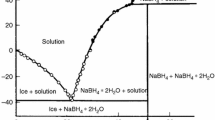
The article describes the thermolysis process of sodium borohydride dihydrate in thermoanalytical experiments. The reaction was carried out without solid catalyst and with catalyst as cobalt boride Co2B. It has been found out that in both cases the process starts after the peritectic reaction of the starting compound and forms a liquid phase. The enthalpy of peritectic reaction is ΔHreact = 19 ± 2 kJ mol−1. When thermolysis proceeds in acetonitrile solution without a catalyst intermediate hydroxyborohydride NaBH3OH and/or Na(BH3)2OH is formed according to the NMR experiment data. The formation of similar complexes in the solid phase is confirmed by experiments on the oxidation of the thermolysis products. Thermolysis process with solid catalyst proceeds with an intense exothermic effect at lower temperatures. The kinetics of the non-catalytic process is described by the model of two consecutive reactions, and reaction with the solid catalyst model is approximated by two parallel reactions.










Similar content being viewed by others
Change history
18 June 2019
The Editor-in-Chief would like to alert readers that due to an administrative error, this article [1] has been republished in the same journal [2]. The correct citations for these articles should be from the original publication [1].
References
Schlesinger HI, Brown HC, Finholt AE, Gilbreath JR, Hoekstra H, Hyde RK. Sodium borohydride, its hydrolysis and its use as a reduction agent and in the generation of hydrogen. J Am Chem Soc. 1953;75:215–9.
Orimo SI, Nakamori Y, Eliseo JR, Zuttel A, Jensen CM. Complex hydrides for hydrogen storage. Chem Rev. 2007;107(10):4111–32.
Amendola SC, Sharp-Goldman SL, Janjua MS, Spencer NC, Kelly MT, Petillo PJ, Binder M. A safe, portable, hydrogen gas generator using aqueous borohydride solution and Ru catalyst. Int J Hydrog Energy. 2000;25(10):969–75.
Marrero-Alfonso EY, Beaird AM, Davis TA, Matthews MA. Hydrogen generation from chemical hydrides. Ind Eng Chem Res. 2009;48:3703–12.
Beaird AM, Davis TA, Matthews MA. Deliquescence in the hydrolysis of sodium borohydride by water vapor. Ind Eng Chem Res. 2010;49:4596–9.
Yongsheng Wei Ru, Wang Liyuan Meng, Wang Yan, Li Guode, Xin Shigang, Zhao Xinsheng, Zhang Ke. Hydrogen generation from alkaline NaBH4 solution using a dandelion-like Co–Mo–B catalyst supported on carbon cloth. Int J Hydrog Energy. 2017;42(15):9945–51.
Li Qiming, Li Fang, Zhao Shiduo, Xia Xin. Hydrogen generation from hydrolysis of NaBH4 based on high stable NiB/NiFe2O4 catalyst. Int J Hydrog Energy. 2017;42(7):3971–80.
Simagina VI, Komova OV, Ozerova AM, Netskina OV, Odegova GV, Kellerman DG, Bulavchenko OA, Ishchenko AV. Cobalt oxide catalyst for hydrolysis of sodium borohydride and ammonia borane. Appl Catal A. 2011;394:86–92.
Malceva NN, Khain VC. Sodium borohydride. Moscow: Nauka; 1985 (in Russian).
Filinchuk Y, Hagemann H. Structure and properties of NaBH4·2H2O and NaBH4. Eur J Inorg Chem. 2008;20:3127–33.
Marrero-Alfonso EY, Gray JR, Davis TA, Matthews MA. Minimizing water utilization in hydrolysis of sodium borohydride: the role of sodium metaborates hydrates. Int J Hydrog Energy. 2007;32:4723–30.
Khain VC, Malceva NN, Volkov AA. Borohydrides of alkali metals and tetraalkylammonium. Ukhta: Ukhta State University; 2001 (in Russian).
Ruman T, Kushnierz A, Jurkiewicz A, Les A, Rode W. The synthesis, reactivity and 1H NMR investigation of the hydroxyborohydride anion. Inorg Chem Commun. 2007;10:1074–8.
Arkhangelsky IV, Dunaev AV, Makarenko IV, Tikhonov NA, Belyaev SS, Tarasov AV. Non-isothermal kinetic methods. Workbook and laboratory. manual ed. Berlin: Open Access; 2013.
Ozawa T. A new method of analyzing thermo gravimetric data. Bull Chem Soc Jpn. 1881;1965:38.
Acknowledgements
This work was supported by the RFBR under Grant No. 15-03-0750.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arkhangelskii, I.V., Tarasov, V.P., Kravchenko, O.V. et al. Thermoanalytical and NMR investigation of NaBH4·2H2O thermolysis process. J Therm Anal Calorim 131, 2833–2842 (2018). https://doi.org/10.1007/s10973-017-6821-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6821-3