Abstract
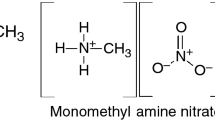
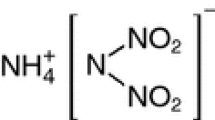
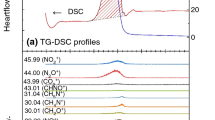
This paper focuses on the thermal behavior of mixtures of ammonium dinitramide (ADN) and amine nitrates. Because some mixtures of ADN and amine nitrate exhibit low melting points and high-energy content, they represent potential liquid propellants for spacecraft. This study focused on the melting behavior and thermal-decomposition mechanisms in the condensed phase of ADN/amine nitrate mixtures during heating. We measured the melting point and exothermal behavior during constant-rate heating using differential scanning calorimetry and performed thermogravimetry–differential thermal analysis–mass spectrometry (TG–DTA–MS) to analyze the thermal behavior and evolved gases of ADN/amine nitrate mixtures during simultaneous heating to investigate their reaction mechanisms. Results showed that the melting point of ADN was significantly lowered upon the addition of amine nitrate with relatively low molecular volume and low melting point. TG–DTA–MS results showed that the onset temperature of the thermal decomposition of ADN/amine nitrates was similar to that of pure ADN. Furthermore, during thermal decomposition in the condensed phase, ADN produced highly acidic products that promoted exothermic reactions, and we observed the nitration and nitrosation of amines from the dissociation of amine nitrates.




Similar content being viewed by others
References
Guerya JF, Chang IS, Shimada T, Glick M, Boury D, Robert E, Napior J, Wardle R, Perut C, Calabro M, Glick R, Habu H, Sekino N, Vigier G, Andrea BD. Solid propulsion for space applications: an updated roadmap. Acta Astronaut. 2010;66:201–19.
Trache D, Klapötke TM, Maiz L, Abd-Elghany M, DeLuca LT. Recent advances in new oxidizers for solid rocket propulsion. Green Chem. 2017;19:4711–36.
Kumar P. An overview on properties, thermal decomposition, and combustion behavior of ADN and ADN based solid propellants. Def Technol. 2018. https://doi.org/10.1016/j.dt.2018.03.009.
Bottaro JC, Penwell PE, Schmitt RJ. 1,1,3,3-Tetraoxo-1,2,3-triazapropene anion, a new oxy anion of nitrogen: the dinitramide anion and its salts. J Am Chem Soc. 1997;119:9405–10.
Pak Z. Some ways to higher environmental safety of solid rocket propellant application. In: Proceedings of the AIAA/SAE/ASME/ASEE 29th joint propulsion conference and exhibition. 1993; AIAA-93-1755.
Östmark H, Bemm U, Langlet A, Sanden R, Wingborg N. The properties of ammonium dinitramide (ADN): part 1, basic properties and spectroscopic data. J Energ Mater. 2000;18:123–8.
Venkatachalam S, Santhosh G, Nian KN. An overview on synthetic routes and properties of ammonium dinitramide (ADN) and other dinitramide salts. Propel Explos Pyrotech. 2004;29:178–87.
Anflo K, Grönland TA, Wingborg N. Development and testing of ADN-based monopropellants in small rocket engines. In: Proceedings of the 36th AIAA/ASME/SAE/ASEE joint propulsion conference, 2000;AIAA-2000-3162.
Negri M, Wilhelm M, Hendrich C, Wingborg N, Gediminas L, Adelow L, Maleix C, Chabernaud P, Brhmi R, Beauchet R, Batonneau Y, Kappenstein C, Koopmans RJ, Schuh S, Bartok T, Scharlemann C, Gotzig U, Schwentenwein M. New technologies for ammonium dinitramide based monopropellant thrusters—the project RHEFORM. Acta Astronaut. 2018;143:105–17.
Matsunaga H, Habu H, Miyake A. Preparation and thermal decomposition behavior of ammonium dinitramide-based energetic ionic liquid propellant. Sci Technol Energ Mater. 2017;78:69–74.
Trache D, Khimeche K, Benelmir R, Dahmani A. DSC measurement and prediction of phase diagrams for binary mixtures of energetic materials’ stabilizers. Thermochim Acta. 2013;565:8–16.
Trache D, Khimeche K, Benziane M, Dahmani A. Solid–liquid phase equilibria for binary mixtures of propellant’s stabilizaers. J Therm Anal Calorim. 2013;112:215–22.
Secordel X, Daigurande D, Beauchet R, Batonneau Y, Kappenstein C, Wingborg N. Calculated and experimental binary phase diagrams for ADN and AN based solid propellant-H2020 GRAIL project. In: Proceedings of the 7th European conference for aeronautics and space sciences (EUCASS). 2017.
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem Commun. 2003;39:70–1.
Wilkes JS. A short history of ionic liquids—from molten salts to neoteric solvents. Green Chem. 2002;4:73–80.
Matsunaga H, Katoh K, Habu H, Noda M, Miyake A. Preparation and thermal decomposition behavior of high-energy ionic liquids based on ammonium dinitramide and amine nitrates. Trans JSASS Aerosp Technol Jpn. 2018;16:88–92.
Shiota K, Izato Y, Matsunaga H, Habu H, Miyake A. Thermal properties of ADN, monomethylamine nitrate and urea based ionic liquid gel propellants. Trans JSASS Aerosp Technol Jpn. 2018;16:93–7.
Shiota K, Matsunaga H, Miyake A. Effects of amino acids on solid-state phase transition of ammonium nitrate. J Therm Anal Calorim. 2017;127:851–6.
Trache D, Khimeche K. Study on the influence of ageing on thermal decomposition of double-base propellants and prediction of their in-use time. Fire Matter. 2013;37:328–36.
Trache D, Khimeche K, Mezroua A, Benziane M. Physicochemical properties of microcrystalline nitrocellulose from Alfa grass fibres and its thermal stability. J Therm Anal Calorim. 2016;124:1485–96.
Yamaki N, Shiota K, Izato Y, Miyake A. Analysis of the thermal hazards of 1-butyl-3-methylimidazolium chloride mixtures with cellulose and various metals. J Therm Anal Calorim. 2018;133:797–803.
Amrousse R, Katsumi T, Azuma N, Hori K. Hydroxylammonium nitrate (HAN)-based green propellant as alternative energy resource for potential hydrazine substitution: from lab scale to pilot plant scale-up. Combust Flame. 2017;176:334–48.
Izato Y, Koshi M, Miyake A, Habu H. Kinetics analysis of thermal decomposition of ammonium dinitramide (ADN). J Therm Anal Calorim. 2017;127:255–64.
Karunakaran K. Theoretical prediction of eutectic temperature and composition. J Sol Chem. 1981;10:431–5.
Oxley JC, Smith JL, Zheng W, Rogers E, Coburn MD. Thermal decomposition studies on ammonium dinitramide (ADN) and 15N and 2H isotopomers. J Phys Chem A. 1997;101:5642–52.
Vyazokin S, Wight CA. Ammonium dinitramide: kinetics and mechanism of thermal decomposition. J Phys Chem A. 1997;101:5653–8.
Löbbecke S, Krause H, Pfeil A. Thermal analysis of ammonium dinitramide decomposition. Propel Explos Pyrotech. 1997;22:184–8.
Kazakov AI, Rubtsov YI, Andrienko LP, Manelis GB. Kinetic of the thermal decomposition of dinitramide 3. Kinetics of the heat release during the thermal decomposition of dinitramide ammonium salt in the liquid phase. Russ Chem Bull. 1998;47:379–85.
Kazakov AI, Rubtsov YI, Manelis GB. Kinetics and mechanism of thermal decomposition of dinitramide. Propel Explos Pyrotech. 1999;24:37–42.
Pavlov AN, Grebennikov VN, Nazina LD, Nazin GM, Manelis GB. Thermal decomposition of ammonium dinitramide and mechanism of anomalous decay of dinitramide salts. Russ Chem Bull. 1999;48:50–4.
Tompa AS. Thermal analysis of ammonium dinitramide (ADN). Thermochim Acta. 2000;357–8:177–93.
Mishra IB, Russell TP. Thermal stability of ammonium dinitramide. Thermochim Acta. 2002;384:47–56.
Shmakov AG, Korobenichev OP, Bol’shova TA. Thermal decomposition of ammonium dinitramide vapor in a two-temperature flow reactor. Combst Explos Shock Waves. 2002;38:284–94.
Matsunaga H, Habu H, Miyake A. Influences of aging on thermal decomposition mechanism of high performance oxidizer ammonium dinitramide. J Therm Anal Calorim. 2013;113:1387–94.
Matsunaga H, Habu H, Miyake A. Thermal behavior of new oxidizer ammonium dinitramide. J Therm Anal Calorim. 2013;111:1183–8.
Matsunaga H, Habu H, Miyake A. Thermal decomposition of the high-performance oxidizer ammonium dinitramide under pressure. J Therm Anal Calorim. 2014;116:1227–32.
Matsunaga H, Izato Y, Habu H, Miyake A. Thermal decomposition characteristics of mixtures of ammonium dinitramide and copper(II) oxide. J Therm Anal Calorim. 2015;121:319–26.
Matsunaga H, Habu H, Miyake A. Analysis of evolved gases during the thermal decomposition of ammonium diniramide under pressure. Sci Tech Energ Mater. 2017;78:81–6.
Jain SR, Rao MV. Thermal reactivity of methylammonium nitrates. Propel Explos Pyrotech. 1978;3:83–7.
Kurniadi W, Brower KR. A reinvestigation of thermal decomposition of methylammonium nitrate. J Org Chem. 1994;59:5502–5.
Izato Y, Miyake A. Thermal decomposition of molten ammonium nitrate (AN). Therm Anal Calorim. 2015;122:595–600.
Williams DLH. Nitrosation reaction and the chemistry of nitric oxide. Amsterdam: Elsevier; 2004.
Sun J, Sun Z, Wang Q, Ding H, Wang T, Jiang C. Catalytic effects of inorganic acids on the decomposition of ammonium nitrate. J Hazardous Matter. 2005;127:204–10.
Suzuki H. A dictionary of nitrogen oxides. Tokyo: Maruzen; 2008 (in Japanese).
Acknowledgements
This work was supported by the JSPS KAKENHI Grant Number 16H06134.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsunaga, H., Katoh, K., Habu, H. et al. Thermal behavior of ammonium dinitramide and amine nitrate mixtures. J Therm Anal Calorim 135, 2677–2685 (2019). https://doi.org/10.1007/s10973-018-7875-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7875-6