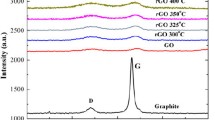
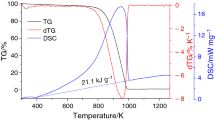
Abstract
Thermal annealing is a convenient, low-cost, and versatile route to fine-tune the physicochemical properties of graphene oxide paper (GOP) toward various applications. Therefore, a thorough monitoring of the thermal decomposition of GOP is generally required for a precise and safe adjustment of its properties. From this perspective, thermogravimetry analysis (TG-A) is employed to scrutinize the multi-step thermal decomposition process of GOP under a nitrogen atmosphere. GOP is elaborated from modified hummers graphene oxide (GO). Nonisothermal TG-A is carried out for the as-prepared GOP at different heating rates. Activation energy is assessed for the overall decomposition process by several isoconversional models. Derivative thermogravimetry (D-TG) curves are derived numerically from TG-A data. To find the number of reactions involved, D-TG curves are deconvoluted into individual peaks by the well-known Fraser–Suzuki function. The obtained results show that the thermal decomposition of GOP consists mainly of six reactions: physisorbed water dehydration, rearrangement and elimination of oxygen functional groups, disproportionation reaction, chemisorbed water dehydration, desulfonation, and degradation of the carbon framework. Furthermore, kinetic triplets for each of the individual reactions are figured out by coupling the Vyazovkin nonlinear method with the compensation effect.













Similar content being viewed by others
References
Liu R, Gong T, Zhang K, Lee C, Marothiya G, Vijay C, et al. Graphene oxide papers with high water adsorption capacity for air dehumidification. Sci Rep. 2019;7:969–74. https://doi.org/10.1016/j.jhazmat.2018.11.100.
Yeh CN, Raidongia K, Shao J, Yang QH, Huang J. On the origin of the stability of graphene oxide membranes in water. Nat Chem. 2015;7:166–70. https://doi.org/10.1038/nchem.2145.
Arul R, Oosterbeek RN, Robertson J, Xu G, Jin J, Simpson MC. The mechanism of direct laser writing of graphene features into graphene oxide films involves photoreduction and thermally assisted structural rearrangement. Carbon. 2016;99:423–31. https://doi.org/10.1016/j.carbon.2015.12.038.
El-Kady MF, Strong V, Dubin S, Kaner RB. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science. 1979;2012(335):1326–30. https://doi.org/10.1126/science.1216744.
Chen CM, Huang JQ, Zhang Q, Gong WZ, Yang QH, Wang MZ, et al. Annealing a graphene oxide film to produce a free standing high conductive graphene film. Carbon. 2012;50:659–67. https://doi.org/10.1016/j.carbon.2011.09.022.
Moon IK, Lee J, Ruoff RS, Lee H. Reduced graphene oxide by chemical graphitization. Nat Commun. 2010;1:73. https://doi.org/10.1038/ncomms1067.
Bae S-Y, Jeon I-Y, Yang J, Park N, Shin HS, Park S, et al. Large-area graphene films by simple solution casting of edge-selectively functionalized graphite. ACS Nano. 2011;5:4974–80. https://doi.org/10.1021/nn201072m.
Ibrahim AFM, Lin YS. Synthesis of graphene oxide membranes on polyester substrate by spray coating for gas separation. Chem Eng Sci. 2018;190:312–9. https://doi.org/10.1016/j.ces.2018.06.031.
Ai X, Zhang P, Dou Y, Wu Y, Pan T, Chu C, et al. Graphene oxide membranes with hierarchical structures used for molecule sieving. Sep Purif Technol. 2020;230:115879. https://doi.org/10.1016/j.seppur.2019.115879.
Akbi H, Rafai S, Mekki A, Touidjine S, Fertassi M, Kadri DE. When Copper Oxide meets graphene oxide: A core-shell structure via an intermittent spray coating route for a highly efficient ammonium perchlorate thermal decomposition. J Organomet Chem. 2022;957:122159.
Akbi H, Rafai S, Mekki A. Reduced graphene oxide film with an ultrahigh energy density for Battery-like supercapacitor. Materials Chemistry. 2021 [cited 2021 Dec 21];21. Available from: https://www.researchgate.net/profile/Mohamed-Kadari/publication/353333687_Proceedings_Book_ISyMC2021/links/60f56d2e16f9f3130093e897/Proceedings-Book-ISyMC2021.pdf#page=42
Akbi H, Yu L, Wang B, Liu Q, Wang J, Liu J, et al. Effect of reducing system on capacitive behavior of reduced graphene oxide film: Application for supercapacitor. J Solid State Chem. 2015;221:338–44.
Akbi H, Rafai S, Mekki A, Touidjine S, Belkadi K, Boudina N, et al. Boosting the storage capacity and the rate capability of flexible graphene film via a nondestructive thermo-chemical reduction. Diam Relat Mater. 2022;129:109338.
Wang Z, Xu D, Huang Y, Wu Z, Wang L, Zhang X. Facile, mild and fast thermal-decomposition reduction of graphene oxide in air and its application in high-performance lithium batteries. Chem Commun. 2012;48:976–8.
Zeng Y, Li T, Yao Y, Li T, Hu L, Marconnet A. thermally conductive reduced graphene oxide thin films for extreme temperature sensors. Adv Funct Mater. 2019;29:1901388. https://doi.org/10.1002/adfm.201901388.
Pham VH, Cuong TV, Hur SH, Shin EW, Kim JS, Chung JS, et al. Fast and simple fabrication of a large transparent chemically-converted graphene film by spray-coating. Carbon. 2010;48:1945–51.
Choi Y-Y, Kang SJ, Kim H-K, Choi WM, Na S-I. Multilayer graphene films as transparent electrodes for organic photovoltaic devices. Solar Energy Mater Solar Cells. 2012;96:281–5. https://doi.org/10.1016/j.solmat.2011.09.031.
Pelaez-Fernandez M, Bermejo A, Benito AM, Maser WK, Arenal R. Detailed thermal reduction analyses of graphene oxide via in-situ TEM/EELS studies. Carbon. 2021;178:477–87.
Chen X, Meng D, Wang B, Li B-W, Li W, Bielawski CW, et al. Rapid thermal decomposition of confined graphene oxide films in air. Carbon. 2016;101:71–6. https://doi.org/10.1016/j.carbon.2016.01.075.
Zhao J, Li Y, Wang Y, Mao J, He Y, Luo J. Mild thermal reduction of graphene oxide as a lubrication additive for friction and wear reduction. RSC Adv. 2017;7:1766–70.
Ju HM, Huh SH, Choi SH, Lee HL. Structures of thermally and chemically reduced graphene. Mater Lett. 2010;64:357–60. https://doi.org/10.1016/j.matlet.2009.11.016.
Le GTT, Manyam J, Opaprakasit P, Chanlek N, Grisdanurak N, Sreearunothai P. Divergent mechanisms for thermal reduction of graphene oxide and their highly different ion affinities. Diam Relat Mater. 2018;89:246–56. https://doi.org/10.1016/j.diamond.2018.09.006.
Sengupta I, Sharat Kumar SSS, Pal SK, Chakraborty S. Characterization of structural transformation of graphene oxide to reduced graphene oxide during thermal annealing. J Mater Res. 2020;35:1197–204. https://doi.org/10.1557/jmr.2020.55.
Hun Seung. Thermal reduction of graphene oxide. In: Mikhailov S, editor. Physics and Applications of Graphene-Experiments. InTech; 2011. https://doi.org/10.5772/14156.
Akbi H, Mekki A, Rafai S, Touidjine S, Boudina N, Sayeh ZB. Phenomenological description of the thermal reduction kinetics in graphene oxide films. Mater Chem Phys. 2022;277:125477.
Rouzière S, Launois P, Benito AM, Maser WK, Paineau E. Unravelling the hydration mechanism in a multi-layered graphene oxide paper by in-situ X-ray scattering. Carbon. 2018;137:379–83.
Núñez JD, Benito AM, Rouzière S, Launois P, Arenal R, Ajayan PM, et al. Graphene oxide–carbon nanotube hybrid assemblies: cooperatively strengthened OH⋯OC hydrogen bonds and the removal of chemisorbed water. Chem Sci. 2017;8:4987–95.
Rouzière S, Núñez JD, Paineau E, Benito AM, Maser WK, Launois P. Intercalated water in multi-layered graphene oxide paper: an X-ray scattering study. J Appl Crystallogr. 2017;50:876–84.
Hummers WS, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958;80:1339–1339. https://doi.org/10.1021/ja01539a017.
Chen J, Yao B, Li C, Shi G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon. 2013;64:225–9. https://doi.org/10.1016/j.carbon.2013.07.055.
Eigler S, Dotzer C, Hof F, Bauer W, Hirsch A. Sulfur species in graphene oxide. Chem-A Eur J. 2013;19:9490–6. https://doi.org/10.1002/chem.201300387.
Shen Y, Boffa V, Corazzari I, Qiao A, Tao H, Yue Y. Revealing hidden endotherm of Hummers’ graphene oxide during low-temperature thermal reduction. Carbon. 2018;138:337–47. https://doi.org/10.1016/j.carbon.2018.05.018.
Eigler S, Grimm S, Hirsch A. Investigation of the thermal stability of the carbon framework of graphene oxide. Chem-A Eur J. 2014;20:984–9. https://doi.org/10.1002/chem.201304048.
Oh YJ, Yoo JJ, Kim YL, Yoon JK, Yoon HN, Kim JH, et al. Oxygen functional groups and electrochemical capacitive behavior of incompletely reduced graphene oxides as a thin-film electrode of supercapacitor. Electrochim Acta. 2014;116:118–28. https://doi.org/10.1016/j.electacta.2013.11.040.
Agarwal V, Zetterlund PB. Strategies for reduction of graphene oxide–A comprehensive review. Chem Eng J. 2021;405:127018. https://doi.org/10.1016/j.cej.2020.127018.
Bottari G, Herranz MÁ, Wibmer L, Volland M, Rodríguez-Pérez L, Guldi DM, et al. Chemical functionalization and characterization of graphene-based materials. Chem Soc Rev. 2017;46:4464–500.
Li C, Lu Y, Yan J, Yu W, Zhao R, Du S, et al. Effect of long-term ageing on graphene oxide: structure and thermal decomposition. R Soc Open Sci. 2021. https://doi.org/10.1098/rsos.202309.
Kim S, Zhou S, Hu Y, Acik M, Chabal YJ, Berger C, et al. Room-temperature metastability of multilayer graphene oxide films. Nat Mater. 2012;11:544–9. https://doi.org/10.1038/nmat3316.
Foller T, Daiyan R, Jin X, Leverett J, Kim H, Webster R, et al. Enhanced graphitic domains of unreduced graphene oxide and the interplay of hydration behaviour and catalytic activity. Materials Today. 2021;50:44–54.
Kim YJ, Kahng YH, Hwang YH, Lee SM, Lee SY, Lee HR, et al. Optimization of graphene oxide synthesis parameters for improving their after-reduction material performance in functional electrodes. Mater Res Express. 2016;3:1–14. https://doi.org/10.1088/2053-1591/3/10/105033.
Jeong H, Lee YP, Jin MH, Kim ES, Bae JJ, Lee YH. Thermal stability of graphite oxide. Chem Phys Lett. 2009;470:255–8. https://doi.org/10.1016/j.cplett.2009.01.050.
Sandoval S, Kumar N, Sundaresan A, Rao CNR, Fuertes A, Tobias G. Enhanced thermal oxidation stability of reduced graphene oxide by nitrogen doping. Chem- A Eur J. 2014;20:11999–2003. https://doi.org/10.1002/chem.201403833.
Krishnan D, Kim F, Luo J, Cruz-Silva R, Cote LJ, Jang HD, et al. Energetic graphene oxide: challenges and opportunities. Nano Today. 2012;7:137–52. https://doi.org/10.1016/j.nantod.2012.02.003.
Kim F, Luo J, Cruz-Silva R, Cote LJ, Sohn K, Huang J. Self-propagating domino-like reactions in oxidized graphite. Adv Funct Mater. 2010;20:2867–73. https://doi.org/10.1002/adfm.201000736.
Qiu Y, Guo F, Hurt R, Külaots I. Explosive thermal reduction of graphene oxide-based materials: Mechanism and safety implications. Carbon. 2014;72:215–23.
Qiu Y, Collin F, Hurt RH, Külaots I. Thermochemistry and kinetics of graphite oxide exothermic decomposition for safety in large-scale storage and processing. Carbon. 2016;96:20–8. https://doi.org/10.1016/j.carbon.2015.09.040.
Lakhe P, Kulhanek DL, Sun W, Zhang B, Green MJ, Mannan MS. Calorimetry of explosive thermal decomposition of graphite oxide. J Hazard Mater. 2019;366:275–81. https://doi.org/10.1016/j.jhazmat.2018.11.100.
Yin K, Li H, Xia Y, Bi H, Sun J, Liu Z, et al. Thermodynamic and kinetic analysis of lowtemperature thermal reduction of graphene oxide. Nanomicro Lett. 2011;3:51–5.
Vyazovkin S, Chrissafis K, di Lorenzo ML, Koga N, Pijolat M, Roduit B, et al. ICTAC kinetics committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Vyazovkin S, Burnham AK, Favergeon L, Koga N, Moukhina E, Pérez-Maqueda LA, et al. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim Acta. 2020;689:178597. https://doi.org/10.1016/j.tca.2020.178597.
Sbirrazzuoli N. Advanced isoconversional kinetic analysis for the elucidation of complex reaction mechanisms: a new method for the identification of rate-limiting steps. Molecules. 2019;24:1683.
Moukhina E. Determination of kinetic mechanisms for reactions measured with thermoanalytical instruments. J Therm Anal Calorim. 2012;109:1203–14. https://doi.org/10.1007/s10973-012-2406-3.
Wada T, Nakano M, Koga N. Multistep kinetic behavior of the thermal decomposition of granular sodium Percarbonate: Hindrance effect of the outer surface layer. J Phys Chem A. 2015;119:9749–60. https://doi.org/10.1021/acs.jpca.5b07042.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19. https://doi.org/10.1016/j.tca.2011.03.034.
Pinzi S, Buratti C, Bartocci P, Marseglia G, Fantozzi F, Barbanera M. A simplified method for kinetic modeling of coffee silver skin pyrolysis by coupling pseudo-components peaks deconvolution analysis and model free-isoconversional methods. Fuel. 2020;278:118260. https://doi.org/10.1016/j.fuel.2020.118260.
Yamamoto K. Thermal decomposition of maya blue: extraction of indigo thermal decomposition steps from a multistep heterogeneous reaction using a kinetic deconvolution analysis. Molecules. 2019;24:2515.
Yan Q-L, Zeman S, Zhang J-G, He P, Musil T, Bartošková M. Multi-stage decomposition of 5-aminotetrazole derivatives: kinetics and reaction channels for the rate-limiting steps. Phys Chem Chem Phys. 2014;16:24282–91.
Brown ME. Introduction to thermal analysis. In: Brown ME, editor. Kluwer, Dodrecht. Dordrecht: Kluwer Academic Publishers; 2004.
Órfão JJM. Review and evaluation of the approximations to the temperature integral. AIChE Journal. 2007;53:2905–15. https://doi.org/10.1002/aic.11296.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Senum GI, Yang RT. Rational approximations of the integral of the Arrhenius function. J Thermal Anal. 1977;11:445–7. https://doi.org/10.1007/BF01903696.
Akbi H, Mekki A, Rafai S. New linear integral kinetic parameters assessment method based on an accurate approximate formula of temperature integral. Int J Chem Kinet. 2022;54:28–41. https://doi.org/10.1002/kin.21538.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Burnham AK, Braun RL. Global kinetic analysis of complex materials. Energy & Fuels. 1999;13:1–22. https://doi.org/10.1021/ef9800765.
Vyazovkin S, Dollimore D. Linear and nonlinear procedures in isoconversional computations of the activation energy of nonisothermal reactions in solids. J Chem Inf Comput Sci. 1996;36:42–5. https://doi.org/10.1021/ci950062m.
Sbirrazzuoli N. Determination of pre-exponential factor and reaction mechanism in a model-free way. Thermochim Acta. 2020;691:178707. https://doi.org/10.1016/j.tca.2020.178707.
Vyazovkin S. Isoconversional Kinetics of Thermally Stimulated Processes. Cham: Springer International Publishing; 2015.
Farivar F, Lay Yap P, Karunagaran RU, Losic D. Thermogravimetric analysis (TGA) of graphene materials: effect of particle size of graphene, graphene oxide and graphite on thermal parameters. C (Basel). 2021;7:41.
Acik M, Lee G, Mattevi C, Pirkle A, Wallace RM, Chhowalla M, et al. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J Phys Chem C. 2011;115:19761–81. https://doi.org/10.1021/jp2052618.
Acik M, Mattevi C, Gong C, Lee G, Cho K, Chhowalla M, et al. The role of intercalated water in multilayered graphene oxide. ACS Nano. 2010;4:5861–8. https://doi.org/10.1021/nn101844t.
Pan Q, Chung C, He N, Jones JL, Gao W. Accelerated thermal decomposition of graphene oxide films in air via in Situ X-ray diffraction analysis. J Phys Chem C. 2016;120:14984–90. https://doi.org/10.1021/acs.jpcc.6b05031.
Klemeyer L, Park H, Huang J. Geometry-dependent thermal reduction of graphene oxide solid. ACS Mater Lett. 2021;3:511–5. https://doi.org/10.1021/acsmaterialslett.0c00423.
Farivar F, Yap PL, Hassan K, Tung TT, Tran DNH, Pollard AJ, et al. Unlocking thermogravimetric analysis (TGA) in the fight against “Fake graphene” materials. Carbon. 2021;179:505–13.
Liu N, Chen H, Shu L, Statheropoulos M. Error evaluation of integral methods by consideration on the approximation of temperature integral. J Therm Anal Calorim. 2005;81:99–105. https://doi.org/10.1007/s10973-005-0751-1.
Sronsri C, Boonchom B. Deconvolution technique for the kinetic analysis of a complex reaction and the related thermodynamic functions of the formation of LiMn 0.90 Co 0.05 Mg 0.05 PO 4. Chem Phys Lett. 2017;690:116–28.
Cheikh Moine E, Groune K, El Hamidi A, Khachani M, Halim M, Arsalane S. Multistep process kinetics of the non-isothermal pyrolysis of Moroccan Rif oil shale. Energy. 2016;115:931–41.
Pomerantsev AL, Kutsenova AV, Rodionova OY. Kinetic analysis of non-isothermal solid-state reactions: multi-stage modeling without assumptions in the reaction mechanism. Phys Chem Chem Phys Royal Soci Chem. 2017;19:3606–15.
Perejón A, Sánchez-Jiménez PE, Criado JM, Pérez-Maqueda LA. Kinetic analysis of complex solid-state reactions. A new deconvolution procedure. J Phys Chem B. 2011;115:1780–91. https://doi.org/10.1021/jp110895z.
Ganguly A, Sharma S, Papakonstantinou P, Hamilton J. Probing the thermal deoxygenation of graphene oxide using high-resolution In Situ X-ray-based spectroscopies. J Phys Chem C. 2011;115:17009–19. https://doi.org/10.1021/jp203741y.
Sengupta I, Chakraborty S, Talukdar M, Pal SK, Chakraborty S. Thermal reduction of graphene oxide: how temperature influences purity. J Mater Res. 2018;33:4113–22. https://doi.org/10.1557/jmr.2018.338.
Acknowledgements
H. AKBI is very indebted to EcoleMilitairePolytechnique, for the provision of PhD Scholarship granted by the project number 15/2020/DRFPG/EMP
Author information
Authors and Affiliations
Contributions
Hamdane AKBI was involved in conceptualization, methodology, investigation, and writing the original draft. Souleyman RAFAI and Ahmed MEKKI were responsible for supervision, conceptualization, methodology, and writing—reviewing and editing. Sabri TOUIDJINE and Kamelia BELKADI took part in characterization and writing—reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akbi, H., Rafai, S., Mekki, A. et al. Kinetic investigation of the multi-step thermal decomposition of graphene oxide paper. J Therm Anal Calorim 148, 3487–3503 (2023). https://doi.org/10.1007/s10973-023-11948-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-11948-1