Abstract
Context
Even though habitat use is essential information for conservation management strategies, studying it in elusive and scarce forest species has proven challenging. Passive acoustic monitoring allows collecting accurate presence-absence data even for species that typically exhibit low detectability. It further provides tools for long-term and cost-effective biodiversity monitoring, and may also be used to infer population estimates at the landscape level.
Objectives
We aimed to demonstrate the application of passive acoustic monitoring to the problem of detecting elusive species, especially in forests, using the Eurasian pygmy owl as a model species. We explored its habitat selection, and estimated occupancy and density at the landscape level in the managed boreal forest.
Methods
We conducted a wide-scale autonomous recording units (ARUs) survey, involving 292 sites in a single season, in a study area covering approx. 370 km2 in south-western Finland. We clustered the detections into territories of males to infer population size by also taking into account the size of home ranges derived from GPS-tags data. Since we were simultaneously monitoring the occupancy of a network of nest boxes and previously estimated the abundance of natural cavities, we could also estimate the proportion of pygmy owls nesting in natural cavities.
Results
Increasing availability of mature forests was consistently the most critical habitat variable both for calling and nesting sites, increasing occupancy probability of the pygmy owls in a landscape dominated by managed forests. The proportion of sites showing occupancy by the pygmy owls was 20.9%, corresponding to an estimate of ca. Six territorial males/100 km2.
Conclusion
Our results confirmed that the pygmy owl can be considered a species of mature and old forests, and we provide the first data-based estimate of the total density of territorial males of this species. Passive acoustic monitoring proved to be an efficient method in detecting the presence of pygmy owls, and may overcome weaknesses of other methods, such as nest box surveys, in order to quantify population numbers.
Similar content being viewed by others
Introduction
The loss and degradation of boreal old-growth forests threaten biodiversity and a wide range of ecosystem services (Betts et al. 2017; Watson et al. 2018). Especially in Europe, the boreal forest has been subject to logging and intense industrial-scale management for wood production, leading to a marked disappearance of old-growth forest (Östlund et al. 1997; Korhonen et al. 2021). Furthermore, as many species are related to old-growth forest attributes, both nest-site losses and habitat degradation are producing a decline of old-growth forest birds (Eggers et al. 2005; Griesser et al. 2007; Björklund et al. 2015; Virkkala 2016). Indeed, if old-growth species occur in managed forests, such habitats are prone to becoming unsuitable for specialists if key resources are removed (Ettwein et al. 2020). Therefore, establishing species-specific ecological requirements is crucial for developing evidence-based conservation strategies (Johnson 1980; Kristan 2006; Apolloni et al. 2018).
Lack of knowledge of habitat use is critical, especially when studying elusive and scarce forest species (e.g., Franklin et al. 2004; Ettwein et al. 2020; Kramer et al. 2021). In recent decades, new non-invasive techniques for monitoring animal populations have been raised as potentially valuable and easy-to-implement tools for collecting extensive data on habitat selection and occupancy (Yule 2000; Burton et al. 2015; Novak et al. 2019). Automated data collection means that surveys can take place simultaneously in different areas, where it would be too expensive to send human observers (Marques et al. 2013). This opportunity allows collecting presence-absence data even for species that typically exhibit low detectability, despite it still does not fully resolve the instances when a species is present and not calling. Using unattended autonomous recording units (ARUs), passive acoustic surveys provide tools for long-term and cost-effective biodiversity monitoring (Sugai et al. 2019). Furthermore, the use of ARUs offers an efficient alternative independent of many of the biases of field surveys, since the technique is non-invasive (minimising the potential effect of the researcher on the behaviour of the target animals), consistent over time, and creates a permanent record of surveys (Brandes 2008; Pérez-Granados et al. 2023).
The use of passive acoustic monitoring introduces methodological questions on the use of vocalizations, i.e., territorial calls, as a substitute for the traditional methods that use the locations of pairs or nesting sites to monitor bird’s habitat selection and occupancy. While nesting sites are typically located in the core area of home ranges, advertising calls may be emitted along the home range boundaries (Burgos and Zuberogoitia 2020). This potential bias may occur especially for birds, e.g., when territorial calls are used for territory mapping. Habitat preferences for core areas surrounding nesting sites and call posts may differ, and it is important to consider the differences between habitat selection for calling and breeding. This evidence is especially relevant for low-density bird species of conservation concern, with typically large home ranges. For low-density predators, it is also essential to produce abundance inferences to assess their population trend and extinction risk. It is also relevant because decision-makers in wildlife policy require reliable population size and density estimates to adopt and justify interventions. Despite the above raised questions, passive acoustic monitoring is still a valuable tool for monitoring the abundance of elusive and low-density bird species, especially because it allows surveying relatively large areas.
The Eurasian pygmy owl Glaucidium passerinum (hereafter pygmy owl) is an elusive predator and forest specialist, occurring at low densities across its distribution range. It is known to be declining in the boreal forest (Korpimäki et al. 2020; Honkala et al. 2021). Previous information about the habitat selection of pygmy owls has been based on nest box data (Morosinotto et al. 2017; Baroni et al. 2021). However, potential sources of bias may arise when dealing only with nest box data: (i) when pairs are nesting in unmonitored natural cavities, they remain undetected (false negatives); (ii) when several boxes are set up within a single home range, only one nest box can be used for nesting and the remaining unused boxes generate absence data despite the owls being present (false negatives); (iii) researchers provide nest boxes, but the habitat where they are placed may not be randomly chosen or it may not reflect the habitat of natural cavities (biased habitat availability). In particular, the number of pairs nesting in natural cavities in nest box areas remains largely unknown, leading to a potential underestimate of the actual number.
Pygmy owls produce readily identifiable sounds, providing an opportunity to use passive acoustic surveys to examine their habitat and to estimate and monitor their abundance. The habitat selection of the sites where spontaneous advertising calling occurs is relevant because territorial animals make a considerable effort in advertising their territory and focus their effort on specific sites rather than randomly within their home range (Delgado and Penteriani 2007; Campioni et al. 2010; Jacobsen et al. 2013). The pygmy owl is a territorial species, as they avoid breeding close to conspecifics (Morosinotto et al. 2017), and engage in strong aggressive behaviour response to simulated intrusions with playback (see, e.g., below). However, the habitat of the sites where they advertise the territory within their home range has, to our knowledge, not been studied.
Our goal here is to use passive acoustic monitoring with a large grid of detectors to overcome different detection errors. Most studies based on passive acoustic surveys conducted in the past have used between one and three ARUs (Sugai et al. 2020), but now the number of studies using sensor arrays of more than 10 recorders is rapidly increasing (e.g., Ross et al. 2018; Roe et al. 2021; Wood et al. 2021a, b). Here, we deployed a wide-scale ARU survey involving 292 sites in a single season. Since we were simultaneously monitoring the occupancy of a network of nest boxes, we could also overcome the bias in nest box surveys by estimating the proportion of pygmy owls nesting in natural cavities. We specifically distinguish habitat preferences based on calls and nest sites, to examine habitat selection of pygmy owls in the different parts of their home-range, thus giving a broader array of conditions that reflect the diversity of habitats used by adults. We also used, for the first time, miniaturised GPS tags on pygmy owl males to verify that the population estimates from passive acoustic survey are consistent with the actual size of home ranges and the potential presence of floaters or bachelor males. Moreover, as one of the main aims of the study is to explore the effects of forest management, we produced forestry data GIS layers to test whether the area of old forests may alter the species occupancy patterns, both for calling and for nesting sites. Finally, the survey allowed us also to better estimate the actual density of territorial pairs or males at the landscape level.
Methods
Study species
The pygmy owl is a forest specialist and an obligate secondary cavity-nester. In the boreal forests of northern Europe, most of pygmy owl nests are located in cavities excavated by great spotted woodpeckers Dendrocopos major or three-toed woodpeckers Picoides tridactylus. Pygmy owls also readily accept woodpecker excavated cavity-type nest boxes for reproduction and food hoarding because they mimic the preferred natural sites (Sonerud et al. 1972; Morosinotto et al. 2017; Masoero et al. 2018; Baroni et al. 2020). So far, available estimates for pygmy owl abundance in Finland have relied on nest box occupancy rates, while the proportion of pairs nesting in tree cavities has remained largely unknown (Morosinotto et al. 2017; Korpimäki et al. 2020).
Passive acoustic monitoring
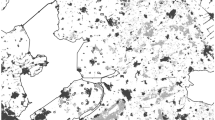
We deployed ARUs, i.e., AudioMoths version 1.1 (Hill et al. 2018), in 292 sites on a regular grid with a spacing of 1 km. We conducted our study in south-western Finland, in the vicinity of Turku (60°N, 22°E), covering approx. 370 km2, mainly consisting of managed Norway spruce (Picea abies) and Scots pine (Pinus sylvestris) forests. We selected every 1-km grid cell from the Finland Uniform Coordinate System (Finnish grid; YKJ, EPSG:2393), and put the recorder within the 100 m radius buffer around the centroid of the grid cell (see Supplementary Material I for examples). If there was no forest within the buffer, we skipped that grid cell (Fig. 1). We conducted the passive acoustic survey between the 16th of March and the 25th of April 2020, when singing activity of pygmy owl peaks and the breeding territories are established (Schönn 1978). ARU devices (AudioMoths) were programmed to record between 00:00 and 7:00 and between 16:00 and 20:00 UTC hours every day, to include the pygmy owl’s calling activity peaks around sunrise and sunset (Mikkola 1970; Schönn 1978), and producing 64 ± 22 h of recordings from every site. We used 90 ARUs, that were progressively moved, using a spatial moving window, so preceding and succeeding adjacent devices were at least partly overlapping their recording schedule.
Detection radius of ARUs
We determined the effective detection radius of the ARUs, to calculate the effective area surveyed and to verify that the same bird calling cannot be detected at the same time by different recorders. We conducted this survey within the study area, in the forest interior, to consider the fact that sound attenuates significantly faster in forests than along roads or forest edges. We first attracted a pygmy owl male at a given location using playback, and recorded it using an Audiomoth attached to a tree at standardised distances ranging from 10 to 500 m. Using a binomial generalised linear model, we analysed how the detection probability of the calls in the spectrogram depended on the distance between the ARU and the advertising owl. We used the likelihood of detection of pygmy owls as a binomial response variable (0 = pygmy owl’s calls not visible in the spectrogram; 1 = pygmy owl’s calls recognisable in the spectrogram), and distance in meters was the explanatory variable. Factors such as the volume of vocalizations (affected by the arousal), perch selection (in the canopy or on tree top), and the precise vegetation conditions can affect the detection radius. However, given the study area’s homogeneous acoustic environment and morphology (the area is flat), we assumed that this single point was representative enough of all 292 survey points for the purpose of verifying whether the same bird call could be detected simultaneously by different ARUs.
Audio analyses
It is still challenging to extract information from acoustic monitoring using signal recognition tools, so as to detect a target species’ occurrence automatically (Brooker et al. 2020). Therefore, we combined three different signal detection and recognition methods:
-
1.
Manual detection
We manually analysed the sonograms of the first two mornings (14 hours of recordings) in every site. Three experienced (or specifically trained) ornithologists (DB, JH and I. Penttinen) viewed the spectrograms and listened to the recordings using the version 3.0.5 of Audacity(R) recording and editing software (Audacity Team 2021) and recorded presence/absence data from these recordings. We did the manual inspection right after collecting the devices from the field, to enable field searching for pairs and nests from sites that detected a male.
-
2.
monitoR
MonitoR (Katz et al. 2016) is an R package offering two template-matching systems for automated detection of acoustic signals. We used the binary-point matching for our analyses. The false positives imply that, after running monitoR on all the recordings, manually checking at least part of the detections is needed, in order to get verified presence/absence data. Therefore, we first run monitoR on the whole dataset of 19 425 hours of recordings, with a score cut-off of 0.4, getting 3 555 935 positive detections. Because of this unexpectedly high number of positive detections and the need of double-checking, we ranked them by the similarity score with the pygmy owl calls, and examined thereafter in spectrograms only the detections with a score > 0.7 in every site, i.e., 6567 detections, to get the true positive detections and filter out the false positive ones.
-
3.
Kaleidoscope pro
Kaleidoscope Pro (v 5.4.2; Wildlife Acoustics Inc, Maynard, USA) uses complex signal processing algorithms based upon Hidden Markov Models to detect and classify syllables based upon their spatio-temporal properties. Detections are grouped into clusters based on their similarity, and each is scored based on its distance from the cluster centre. Lower scores indicate better matches to the training data. We manually reviewed and labelled the clusters and built an advanced classifier designed to eliminate as many false-positive identifications as possible for a single target species recognition. We finally checked all the detections to get the true-positive detection and filter out the false positive ones.
Nest box data and searching of nests
In addition to the passive acoustic survey, we monitored nest boxes to compare nest box occupancy with passive acoustic monitoring. In 2014–2021 there were 121–234 (mean = 198) nest boxes annually; in the study year 2020, the number was 220 (see Baroni et al. 2020 for details).
After checking the nest boxes for nests and visually inspecting the recordings of first two mornings (see above), we got preliminary information about potential territories in which different males from those breeding in nest boxes were advertising. We conducted these playback surveys in areas where positive detections had been previously recorded. To ensure that the male detected during the survey was not the same male breeding in a nearby nest box, we used information on home-range size to select positive detections that were far enough away from known nest boxes. In cases where we were unsure if the detection at a site was the same male nesting in the nest box, we attempted to capture the owls at both sites and used leg-rings for individual recognition. Once we found “new” pairs not breeding in nest boxes, we also extensively searched for their nests in the field.
GPS-tags data
During the breeding seasons 2021–2022, we tagged 14 males and collected movement data from 13 of them (note that the breeding survey was done in 2020). Using a backpack Teflon harness, we equipped 14 males with PinPoint 10 or 50 (Lotek) gps-loggers. Devices (including the harness) weighed 2.91 ± 0.46 g, on average accounting for 5.0 ± 0.8% of the body mass. This is more than is recommended for long-term tagging studies, which is why we only used the tags for a short period and took them away for each individual after 10.3 ± 4.8 days (min. 5, max. 24 days). The owls were caught by mist netting in the same study area of the passive acoustic monitoring. To identify areas exploited by tracked birds, we calculated for each individual the Utilization Distribution (UD) using 90% autocorrelated kernel density estimators (AKDE) by means of the R package ctmm (Calabrese et al. 2021). In 2021 the tags were deployed to males at their known nest sites. In 2022, we deployed tags to males with unknown nest site and breeding status, to assess the proportion of breeding pairs among them, and the potential presence of floaters or bachelor males, which could affect the population estimate inferred from passive acoustic survey.
Habitat data
We calculated the biomass of the foliage (10 kg/ha) of spruce (variable 1) and pine trees (variable 2), i.e., the two dominant tree species in the study area. The two variables were calculated from 2013 Multi-source National Forest Inventory provided by the National Resources Inst. Finland (Mäkisara et al. 2016). Ages of the forest stands were calculated manually, drawing the polygons of clear-cutting forests in the last 80 years. For this purpose, we compared historical aerial photographs from 1949 (available on the website https://kartta.paikkatietoikkuna.fi/) or Google satellite images. We were able to classify all the forest area into the following three variables: (3) clear-cuts and young stands (forest age < 15 years), (4) forest areas (forest age > 15 and < 80 years), (5) and mature forest (forest age > 80 years old). See Suppl. Mat. for a map showing different proportions of forest age in the study area. In addition, we included (6) peatland areas from National Resources Institute Finland and (7) agricultural areas from CORINE land cover. Finally, we calculated a distance in meters for every site from (8) the nearest main road, and (9) the nearest inhabited house. The distances from the ARU to the closest habitat features (variables 8–9) were measured using QGIS tools (QGIS Development Team 2020).
Habitat use analyses
We analysed whether the site occupancy of the pygmy owls was affected by habitat proportions surrounding the sites of the passive acoustic monitoring at three different scales (100, 400 and 1000 m). We used (i) a 100 m radius buffer around each ARU, approximating the minimum detection range of an ARU to detect pygmy owls calling, (ii) a 400 m radius buffer, which still avoids overlaps between different buffers, and (iii) a 1 km radius buffer, which would cover a potential home range if the centre would be at the device. We used binomial generalised linear models (GLM) to analyse the relationship between the habitat variables and the site occupancy by pygmy owls. Occupancy of each site of the passive acoustic monitoring where we placed an ARU was the response variable (0 = pygmy owl not detected, 1 = pygmy owl detected). We included in the model all the habitat variables listed above, as they characterise the habitat surrounding the surveyed sites and overall the whole study area, therefore potentially affecting the habitat selection by pygmy owls. Before selecting the variables to be included in the model, we preliminary checked collinearity of the predictors by correlation coefficient and variance influence factor (VIFs), using the package ‘usdm’ (Naimi 2015) in R version 3.6 (R Core Team 2017). We also centred the variables (mean = 0), before fitting logistic regression using the R package spaMM (Rousset 2021). The explanatory variables were the biomass of the tree foliage within the fixed radius buffer (variables 1–2), the area of variables 3–6 in hectares within the same radius, and the distance in meters (variables 8–9).
We also used the data from the passive acoustic monitoring, and the nest box and playback surveys to construct potential home ranges of known pairs. We used the average size, which we got from GPS-tag data, to construct circular “home ranges” with the nest in the centre. For pairs for whom the breeding sites were not found in the field, we used the location of the pairs or the geometric centre of the positive detections. Finally, we compared the habitat proportions inside these 22 “home ranges” with 100 randomly placed “home ranges” of the same size where the pygmy owl is not present, to describe home range level habitat selection. We thus used a binomial generalised linear model (GLM) with the status of the home range as a response variable (1 = occupied, 0 = not occupied) and the same habitat predictors used for the other models.
Results
Acoustic recogniser performance
The three methods did not entirely agree when concerning the presence/absence of pygmy owl males during the recording period, and hence it was necessary to combine their results. Indeed, each method alone would have allowed us to find only 55.7–62.3% of the presence sites, with Kaleidoscope having slightly lower performance than monitoR and visual inspection. Moreover, manual post-processing of the detections was essential due to very high false-positive rates of Kaleidoscope and monitoR, i.e. 79.0 and 72.8% of the presence sites, respectively. A more detailed evaluation section of different methods is available in the supplementary material.
The accumulation curve shows that the detection probability of pygmy owls was stable in time, with wide CIs in the beginning showing only initial uncertainty (Fig. 2). The ARUs were set up in the field for 5.9 ± 2.0 days, while the species was detected within the first two days (22 h of recordings) after deployment in 80.8% of presence sites. In the remaining 19.2% of presence sites, the first detection of the species was between the third and the fifth day after ARU deployment, and no new first-detections were observed on days 6 and 7.
Detection radius of ARUs
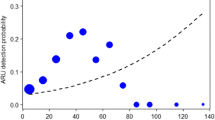
The pygmy owl detection probability in the ARUs was high up to 100–150 m and after that rapidly decreased, dropping to zero at more than 250 m (Distance (m) log-odd estimate with SE: -0.044 ± 0.020, P = 0.03, Fig. 3). If we assume a buffer of at least 150 m radius around the recorders, within which the advertising males are always detected, the passive acoustic survey with 292 ARU effectively monitored an area of 20.64 km2. Also, the distance between the recorders was large enough to ensure that a single bird cannot be detected at the same time by different recorders.
Pygmy owl occurrence in relation to habitat
The results of binomial generalised linear models showed that the probability of pygmy owl occurrence in a habitat increased with the proportion of mature forest in all the three models corresponding to buffers with a radius of 100, 400 and 1000 m around ARUs (Table 1, Fig. 4). We found no significant associations between the presence of advertising pygmy owl males and any other habitat variable (Table 1). The same result was shown by the model of home range level habitat selection, with a probability of a potential home range to be occupied increasing with the proportion of old forest in a radius of 1000 m around the nesting site (Fig. 5; Table 2).
Definition of the home ranges
Based on GPS data, the mean home range (90% AKDE) size was 3.99 ± 1.87 km2 (tracking days: 7.1 ± 1.5; N = 13 males). This result implies that in our study design, in every home range, there were on average at least 3–4 ARUs. Also, pygmy owls performed relatively long hunting trips (5.93 ± 1.98 km per day). The maximum distances of the males from the nest sites were 1.54 ± 0.3 km (range of the maximum distance in every home range: 1.0–2.0 km). During the breeding season 2022, we managed to recapture five of the six GPS-tagged males. For three of them we found the nest site in tree cavities, but for the other two males we did not manage to find their nest. However, one of them was paired as we repeatedly saw the male–female interactions. In the other home-range, there was a dead female inside a nest box at the beginning of the breeding season. As the pairs are forming during the previous autumn, it is likely that a female dying in the months before the nesting period cannot be replaced by another one, so that the male failed to breed because of this circumstance. Therefore, despite we did not find the nest, also these two males were territorial despite the possibility that they may have failed their breeding attempts.
Site occupancy and population size estimation
The proportion of ARU sites showing occupancy by the pygmy owls was 20.9% (N = 61 from 292). Determining territories or the number of breeding pairs from these data was more challenging than expected. It was not as easy as we anticipated to cluster the detections to different advertising males. Also, sites with positive detections were quite far from known nests. The closest recorder to an active nest was 390 ± 102 m from the nest (N = 7), while the distance from the nest to the closest recorder with a positive pygmy owl detection was 764 ± 436 m. Indeed, the ARU that was closest to a known nest site in several cases (4/7) failed to detect the presence of the pygmy owl.
While the ARU survey did not provide a simple way of estimating the number of different males, we attempted to estimate pygmy owl abundance in the study area by combining the different methods we used: we first identified the known nest sites, and other pairs from their responses to the playback. We finally combined the information on the home range size and overlap in timing of advertising calls to estimate the sum of breeding pairs and unpaired territorial males (which we, in most cases, cannot separate). To this end, we clustered the detections based on the distance, and finally estimated 22–27 territories fully or partially within the study area (Figs. 4, 6). A proportion of 25–32% of the estimated number of territorial males was thus breeding in nest boxes, while the remaining males were either nesting in tree cavities or remained unpaired (Table 3). This estimate is well in line with the result that 31.1% (19 sites) of the positive ARU detections were within a radius of 2 km from a known nest site (N = 7).
Detections (coloured dots) and non-detections (grey dots) of pygmy owls in the passive acoustic monitoring. The numbers indicate the criteria used for assignments of territories: 1. distance from known nest sites < 2 km; 2. confirmed using the playback method in June; 3. distance from other detections > 3 km; 4. timings of singing events are overlapping with timings of neighbouring males (the line segments join the site detections within every different territory)
Discussion
Our results with habitat data at three spatial scales, and for both calling and nesting sites, show that increasing proportion of mature and old forests was consistently the most important habitat variable increasing occupancy probability of the pygmy owls in a landscape dominated by managed forests. The proportion of sites occupied by the pygmy owls was 20.9%, which is higher than might have been expected, as our study species is rare in the landscape and its home ranges were assumed to be smaller than they actually are. When we clustered the detections into territories, we estimated a density of territorial males that was altogether three times the number of pairs breeding in nest boxes. Considering the estimated number of natural cavities and the number of installed nest boxes (Table 3), we however estimate that pygmy owls preferred nest boxes over natural cavities for breeding.
Importance of mature forests for the pygmy owls
Our results showed that the probability of pygmy owl occurrence, as detected by ARUs, increased with the proportion of old forest in all the radii of 100, 400 and 1000 m around the ARUs. The same result was found on the home range level habitat selection, with probability of a potential home range to be occupied increasing with proportion of old forest in a radius of 1000 m around the nesting site. Previous studies on habitat choice of pygmy owls in the boreal forest clearly indicate the importance of spruce forest and the preference for large forested areas (Morosinotto et al. 2017; Baroni et al. 2021). Moreover, the importance of the age of the forest has been already highlighted by Morosinotto et al. (2017), who found that the proportion of old spruce and old pine forests increased the probability of nest box occupancy. Our results strongly support the importance of the age of the forests, and pygmy owls can thus be considered mature and old forest specialists. As a mature forest specialist, the pygmy owl may be therefore particularly threatened by the impact of forest management on landscape age structure. Its occurrence in the managed boreal forest in Finland shows that it is not restricted to pristine or protected forests without ongoing forest management. However, our results showed that for pygmy owls, a proportion of mature forests is needed continuously in a forest landscape. The maintenance of a sufficient proportion of mature/old forest is essential for providing both nesting and foraging habitats for most of the threatened old forest specialists (Pakkala et al. 2018a, b; Ettwein et al. 2020). The development of passive acoustic surveys for detecting old-growth forest specialists can potentially help forest management in this area of the world which has some unique challenges. Our approach can be used to identify forest patches with high species richness of mature forest specialists for conservation or management purposes. In our study system, passive acoustic survey allowed to estimate the occupancy of pygmy owls at the landscape level, and if these results are combined with the nest survey, they provided crucial information to implement large-scale habitat management and conservation.
Home range and territory use and properties
The interpretation of the results from the passive acoustic survey needs to take into account that close to the nesting sites, pygmy owls' calls may not be recorded because the owls are not necessarily advertising there. Therefore, we also analysed the home range scale habitat selection for nesting sites. Home ranges have nests in a central location, from which the owl moves in all directions for hunting across large forest areas. We found that pygmy owl home ranges are more extensive than previously thought (Strøm and Sonerud 2001; Barbaro et al. 2016), supporting the need to evaluate conservation measures at the landscape scale. Our data also indicated that males defend their own territory by advertising close to the territory’s borders. This behaviour was already known for other owl species, with vocal activity being higher closer to the home range border (Burgos and Zuberogoitia 2020). Advertising requires significant energy expenditures and a territory owner must thus select the most strategic sites as song posts. Pygmy owls’ advertising calls are not loud compared to the large area of their home ranges, and males may therefore need to use different song posts along the boundaries to deliver the signal and detect vocal responses from neighbouring males. Moreover, to remain silent close to the nesting site may be an anti-predator behaviour. We suggest that this behaviour resulted in negative detections in most of the monitoring sites that were actually located within the core area of the territories, so that the species was actually present but silent in the sites used for hunting or for nesting but not for advertising, and therefore not detectable by ARUs. Further evidence for this hypothesis is provided by no nesting sites being found in the forest patches where the species was detected from the passive acoustic survey.
Higher detection probability close to the territory boundaries is a potential source of bias for many different methods and taxa, e.g., when territorial markings are used as species presence data for spatial ecology studies (López-Bao et al. 2018; Sittenthaler et al. 2020; Ruprecht et al. 2021). This may be true for both scent-marking carnivores (Darden et al. 2008; Monclús et al. 2009; Vogt et al. 2014) as well as for territorial birds, when territorial calls are used for territory mapping. Interestingly, we found a substantial positive effect of the age of the forest on pygmy owl probability of occupancy at all spatial scales and both for calling and for nesting sites. Thus, the pygmy owl male shows a clear habitat preference for using old forests for advertising. It is also possible that the male in the spring is advertising many different sites, from which the female then chooses the nest site.
How many pygmy owls are there?
Passive acoustic monitoring proved to be a very effective method to detect the pygmy owls in sites where they regularly advertise for territorial defence, resulting in a high detectability in only two days and all individuals being detected in five days (as no new site detections were made in days 6 and 7). However, determining territories or the number of breeding pairs from these data was more challenging than expected. We nonetheless managed to extrapolate the number of males by combining information of how many nests we had in nest boxes, how many different pairs and territorial males we found with playback survey in June, by checking which singing events were overlapping in time at different ARUs, and by utilising home range size from GPS-tracking data. As a result, we finally extrapolated a density of roughly six male territories fully or partially in the 100 km2 study area. They included seven pairs nesting in nest boxes, four verified pairs and two territorial males found during the playback survey in June, and another 9–14 males estimated from passive acoustic monitoring data only (Figs. 4, 6). As there may be uncertainty in identifying territories, we decided to provide a range rather than an exact estimate of their number. However, it has been found that the extent over which owls typically vocalize is small relative to space use, such that the likelihood of double counting neighbouring males is low (Reid et al. 2021).
Our attempt to calculate the density of pygmy owls at the landscape scale is the first that has not been based on a playback survey and that includes also both the proportion of pairs not nesting in nest boxes and the potential non-breeding males (Korpimäki et al. 2020; Honkala et al. 2021). It turned out by extrapolation that nest boxes are strongly preferred to tree cavities for nesting, although the cavities were estimated to be 16 times more abundant in the study area (Baroni et al. 2020). The resulting estimate of occupancy by nesting pygmy owls would be on average approximately 0.6% for tree cavities, which is in line with the few nests we have found over the few years when examining occupancy of between 88 and 143 woodpecker cavities. This proportion is lower than the calculated occupancy of 4.1% in nest boxes. The proportion may be even more skewed if some of the territorial males detected in the passive acoustic monitoring were unpaired. However, on the basis of the data collected GPS-tagging the males with unknown breeding status, most of those territories where we did not confirm the presence of a female could host a breeding pair. Indeed, as the pygmy owl is a short-lived species, the proportion of non-breeding individuals is expected to be low because of the risk of dying before the next chance of reproducing (Shaw and Levin 2013; Öst et al. 2018). Therefore, even if the nest boxes are preferred over the tree cavities, the latter are playing the major role in sustaining the population.
Methodological advantages and challenges of the passive acoustic survey
Simultaneous and continuous recordings increase the probability of detection even for low-density and elusive species with low singing rates, such as pygmy owls. Previous existing information from acoustic surveys using readily-available signal recognition tools for use in biodiversity surveys have typically used few hours of recordings for testing (Brooker et al. 2020). However, our survey proves that even less than one false positive detection per day created big problems in dealing with too many false detections, even in sites where the target species was absent. Even improved recognizers usually correctly identify only up to 59% or 74% of individual singing events of a target species (Brooker et al. 2020).
Our results show that, at least for our target species, false-negative and false-positive rates are still very high when employing two of the most commonly used automated approaches, i.e. monitoR and Kaleidoscope. Visual inspection of the spectrogram was, in our case, the best but very time-consuming method, and, therefore, it may not be the most effective approach in terms of vocalizations detected per unit of effort. Therefore, it was worth using multiple methods, but more work is needed to improve deriving presence-absence from the recordings, which currently requires intense manual verification effort. In other previous studies implementing passive acoustic monitoring on owl populations, it has been either needed to manually review audio data for additional calls missed by the detector (Wood et al. 2019, 2021a; b; Reid et al. 2021), or avoided automated detectors to use only the manual detection of calls (Astaras et al. 2022). Additionally, we successfully validated MonitoR operator-verified detections using an approach based on one-third octave band coefficients measured over spectra derived across the recordings (Supplementary Material).
Although false negatives are a common bias in passive acoustic surveys for most study systems, we did not encounter this issue in our study since the detection probability was high. However, in cases where detection probability is low, occupancy models can be a valuable tool to account for imperfect detection and correct for false negatives. Passive acoustic surveys can readily generate all the necessary elements for these models (MacKenzie et al. 2002; Tyre et al. 2003; Wood and Peery 2022). Thus, we recommend the use of occupancy modelling in future studies similar to ours, where false negatives may be present. Furthermore, in our study, we classified a site as occupied when we detected at least one vocalization of the pygmy owl. However, vocal behaviour and intensity of use, such as vocal rate, have also been used to define acoustic detections and accurately classify sites as truly occupied, which can aid in interpreting the occupancy patterns (Reid et al. 2021; Wood and Peery 2022).
Conclusion
Overall, our study demonstrates the potential of passive acoustic monitoring to overcome biases present in other methods estimating both the occupancy and population abundance. This approach proved to be an efficient method in detecting the presence of pygmy owls. By deploying a large number of passive acoustic monitoring devices, a large area can be covered simultaneously, which is needed to detect this bird of prey occurring in low densities in forested landscapes. However, it remains essential to enhance the effectiveness of automated recognition software, for example, by utilizing recently developed classifiers that use convolutional neural network algorithms (Kahl et al. 2021; Perez-Granados, 2023). Regarding the estimate of population abundance, the passive acoustic monitoring may overcome bias of other methods, such as the nest survey, as we did manage to estimate a quite large proportion of the population not included in the nest box monitoring. However, in order to quantify population numbers, we found that a deep knowledge of the spatial ecology of the target species, such as their home range size, is needed for correct assignment of detections to different individuals.
While some previous studies have suggested that pygmy owls are old forest specialists, our study adds several novel contributions to this existing knowledge, especially on the relationship between calling behaviour and nest sites. We also described for the first time the habitat preferences for the sites used for calling sites. Taken together, our findings underline that pygmy owl is an old forest species that is scarce in the landscape, even though more common than could be concluded from a nest box study only. This is essential information for conservation and it indicates the utility of passive acoustic surveys in collecting such data, although the methodology still requires further testing and validation with several methods.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Apolloni N, Grüebler MU, Arlettaz R, Gottschalk TK, Naef-Daenzer B (2018) Habitat selection and range use of little owls in relation to habitat patterns at three spatial scales. Anim Conserv 21(1):65–75
Astaras C, Valeta C, Vasileiadis I (2022) Acoustic ecology of tawny owl (Strix aluco) in the Greek Rhodope mountains using passive acoustic monitoring methods. Folia Oecol 49(2):110–116
Audacity Team (2021) Audacity(R): free audio editor and recorder [Computer application]. Version 3.0.5. https://audacityteam.org/
Barbaro L, Blache S, Trochard G, Arlaud C, de Lacoste N, Kayser Y (2016) Hierarchial habitat selection by Eurasian Pygmy Owls Glaucidium passerinum in old- growth forests of the southern French Alps. J Ornithol 157:333–342
Baroni D, Korpimäki E, Selonen V, Laaksonen T (2020) Tree cavity abundance and beyond: nesting and food storing sites of the pygmy owl in managed boreal forests. For Ecol Manag 460:117818
Baroni D, Masoero G, Korpimäki E, Morosinotto C, Laaksonen T (2021) Habitat choice of a secondary cavity user indicates higher avoidance of disturbed habitat during breeding than during food-hoarding. For Ecol Manag 483:118925
Betts MG, Wolf C, Ripple WJ, Phalan B, Millers KA, Duarte A, Butchart SHM, Levi T (2017) Global forest loss disproportionately erodes biodiversity in intact landscapes. Nature 547:441–444
Björklund H, Valkama J, Tomppo E, Laaksonen T (2015) Habitat effects on the breeding performance of three forest-dwelling hawks. PLoS ONE 10(9):e0137877
Brandes TS (2008) Automated sound recording and analysis techniques for bird surveys and conservation. Bird Conserv Int 18(S1):S163–S173
Brooker SA, Stephens PA, Whittingham MJ, Willis SG (2020) Automated detection and classification of birdsong: an ensemble approach. Ecol Indic 117:106609
Burgos G, Zuberogoitia I (2020) A telemetry study to discriminate between home range and territory size in Tawny Owls. Bioacoustics 29(1):109–121
Burton AC, Neilson E, Moreira D, Ladle A, Steenweg R, Fisher JT, Bayne E, Boutin S (2015) Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. J Appl Ecol 52(3):675–685
Calabrese JM, Fleming CH, Noonan MJ, Dong X (2021) Ctmmweb: a graphical user interface for autocorrelation-informed home range estimation. Wildl Soc Bull 45(1):162–169
Campioni L, Delgado MM, Penteriani V (2010) Social status influences microhabitat selection: breeder and floater Eagle Owls Bubo bubo use different post sites. Ibis 152:569–579
Darden SK, Steffensen LK, Dabelsteen T (2008) Information transfer among widely spaced individuals. Latrines as a basis for communication networks in the swift fox? Anim Behav 75:425–432
Delgado MM, Penteriani V (2007) Vocal behaviour and neighbour spatial arrangement during vocal displays in eagle owls (Bubo bubo). J Zool 271:3–10
Eggers S, Griesser M, Andersson T, Ekman J (2005) Nest predation and habitat change interact to influence Siberian jay numbers. Oikos 111(1):150–158
Ettwein A, Korner P, Lanz M, Lachat T, Kokko H, Pasinelli G (2020) Habitat selection of an old-growth forest specialist in managed forests. Anim Conserv 23(5):547–560
Franklin AB, Gutierrez RJ, Nichols JD, Seamans ME, White GC, Zimmerman GS, Hines JE, Munton TE, LaHaye WS, Blakesley JA (2004) Population dynamics of the California spotted owl (Strix occidentalis occidentalis): a meta-analysis. Ornitholog Monographs. https://doi.org/10.2307/40166799
Griesser M, Nystrand M, Eggers S, Ekman J (2007) Impact of forestry practices on fitness correlates and population productivity in an open-nesting bird species. Conserv Biol 21(3):767–774
Hill AP, Prince P, Piña Covarrubias E, Doncaster CP, Snaddon JL, Rogers A (2018) AudioMoth: evaluation of a smart open acoustic device for monitoring biodiversity and the environment. Methods Ecol Evol 9(5):1199–1211
Honkala J, Piha M, Saurola P, Valkama J (2021) Breeding and population trends of common raptors and owls in Finland in 2020. Linnut-Vuosikirja 2020:70–85
Jacobsen LB, Sunde P, Rahbek C, Dabelsteen T, Thorup K (2013) Territorial calls in the Little Owl (Athene noctua): spatial dispersion and social interplay of mates and neighbours. Ornis Fenn 90(1):41–49
Johnson DH (1980) The comparison of usage and availability measurements for evaluating resource preference. Ecology 61:65–71
Kahl S, Wood CM, Eibl M, Klinck H (2021) BirdNET: a deep learning solution for avian diversity monitoring. Ecol. Inform. 61:101236
Katz J, Hafner SD, Donovan T (2016) Assessment of error rates in acoustic monitoring with the R package monitoR. Bioacoustics 25:177–196
Korhonen KT, Ahola A, Heikkinen J, Henttonen HM, Hotanen JP, Ihalainen A, Melin M, Pitkänen J, Räty M, Sirviö M, Strandström M (2021) Forests of Finland 2014–2018 and their development 1921–2018. Silva Fenn. 55(5):10662
Korpimäki E, Hongisto K, Masoero G, Laaksonen T (2020) The difference between generalist and specialist: the effects of wide fluctuations in main food abundance on numbers and reproduction of two co-existing predators. J Av Biol 51(8):1–13
Kramer A, Jones GM, Whitmore SA, Keane JJ, Atuo FA, Dotters BP, Sawyer SC, Stock SL, Gutiérrez RJ, Peery MZ (2021) California spotted owl habitat selection in a fire-managed landscape suggests conservation benefit of restoring historical fire regimes. For Ecol Manag 479:118576
Kristan WBII (2006) Sources and expectations for hierarchical structure in bird-habitat associations. The Condor 108(1):5–12
López-Bao JV, Godinho R, Pacheco C, Lema FJ, García E, Llaneza L, Palacios V, Jiménez J (2018) Toward reliable population estimates of wolves by combining spatial capture-recapture models and non-invasive DNA monitoring. Sc Rep 8(1):1–8
MacKenzie DI, Nichols JD, Lachman GB, Droege S, Andrew Royle J, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255
Mäkisara K, Katila M, Peräsaari J, Tomppo E (2016) The multi-source national forest inventory of Finland—methods and results 2013.
Marques TA, Thomas L, Martin SW, Mellinger DK, Ward JA, Moretti DJ, Harris D, Tyack PL (2013) Estimating animal population density using passive acoustics. Biol Rev 88(2):287–309
Masoero G, Morosinotto C, Laaksonen T, Korpimäki E (2018) Food hoarding of an avian predator: sex- and age-related differences under fluctuating food conditions. Behav Ecol Sociobiol 72(10):159
Mikkola H (1970) On the activity and food of the Pygmy Owl Glaucidium passerinum during breeding. Ornis Fenn 47:10–14
Monclús R, Arroyo M, Valencia A, De Miguel FJ (2009) Red foxes (Vulpes vulpes) use rabbit (Oryctolagus cuniculus) scent marks as territorial marking sites. J Ethol 27:153–156
Morosinotto C, Villers A, Thomson RL, Varjonen R, Korpimäki E (2017) Competitors and predators alter settlement patterns and reproductive success of an intraguild prey. Ecol Monographs 87(1):4–20
Naimi B (2015) Usdm: uncertainty analysis for species distribution models. R package version 1.1–15. https://CRAN.Rproject.org/web/packages/usdm/usdm.pdf.
Nowak MM, Dziób K, Bogawski P (2019) Unmanned aerial vehicles (UAVs) in environmental biology: a review. Eur J Ecol 4(2):56–74
Öst M, Lindén A, Karell P, Ramula S, Kilpi M (2018) To breed or not to breed: drivers of intermittent breeding in a seabird under increasing predation risk and male bias. Oecologia 188(1):129–138
Östlund L, Zackrisson O, Axelsson AL (1997) The history and transformation of a Scandinavian boreal forest landscape since the 19th century. Can J for Res 27:1198–1206
Pakkala T, Tiainen J, Piha M, Kouki J (2018a) How important are nest cavities made by the three-toed Woddpecker Picoides Tridactylus for cavity-nesting forest bird species? Acta Ornithol 53(1):69–79
Pakkala T, Tiainen J, Piha M, Kouki J (2018b) Three-toed Woodpecker cavities in trees: a keystone structural feature in forests shows decadal persistence but only short-term benefit for secondary cavity-breeders. For Ecol Manag 413:70–75
Pérez-Granados C (2023) BirdNET: applications, performance, pitfalls and future opportunities. Ibis Early View. https://doi.org/10.1111/ibi.13193
QGIS Development Team (2020) QGIS geographic information system. Open source geospatial foundation project. http://qgis.osgeo.org
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reid DS, Wood CM, Whitmore SA, Berigan WJ, Keane JJ, Sawyer SC, Peery MZ (2021) Noisy neighbors and reticent residents: distinguishing resident from non-resident individuals to improve passive acoustic monitoring. Glob. Ecol. Conserv. 28:e01710
Roe P, Eichinski P, Fuller RA, McDonald PG, Schwarzkopf L, Towsey M, Truskinger A, Tucker D, Watson DM (2021) The Australian acoustic observatory. Methods Ecol Evol 12(10):1802–1808
Ross SRJ, Friedman NR, Dudley KL, Yoshimura M, Yoshida T, Economo EP (2018) Listening to ecosystems: data-rich acoustic monitoring through landscape-scale sensor networks. Ecol Res 33:135–147
Rousset MF (2021) Package ‘spaMM’.
Ruprecht JS, Eriksson CE, Forrester TD, Clark DA, Wisdom MJ, Rowland MM, Johnson BK, Levi T (2021) Evaluating and integrating spatial capture-recapture models with data of variable individual identifiability. Ecol Appl 31(7):e02405
Schönn S (1978) Der Sperlingskauz. Die Neue Brehm-Bücherei, Lutherstadt Wittenberg
Shaw AK, Levin SA (2013) The evolution of intermittent breeding. J Math Biol 66:685–703
Sittenthaler M, Schöll EM, Leeb C, Haring E, Parz-Gollner R, Hackländer K (2020) Marking behaviour and census of Eurasian otters (Lutra lutra) in riverine habitats: what can scat abundances and non-invasive genetic sampling tell us about otter numbers? Mammal Res 65(2):191–202
Strøm H, Sonerud GA (2001) Home range and habitat selection in the Pygmy Owl Glaucidium passerinum. Ornis Fenn 78:145–158
Sonerud GA, Mjelde A, Prestrud K, (1972) Spurveuglehekking i fugleholk. Sterna 11:1–12
Sugai LSM, Silva TSF, Ribeiro JW Jr, Llusia D (2019) Terrestrial passive acoustic monitoring: review and perspectives. Bioscience 69(1):15–25
Sugai LSM, Desjonqueres C, Silva TSF, Llusia D (2020) A roadmap for survey designs in terrestrial acoustic monitoring. Remote Sens Ecol Conserv 6(3):220–235
Tyre AJ, Tenhumberg B, Field SA, Niejalke D, Parris K, Possingham HP (2003) Improving precision and reducing bias in biological surveys: estimating false-negative error rates. Ecol Appl 13:1790–1801
Virkkala R (2016) Long-term decline of southern boreal forest birds: consequence of habitat alteration or climate change? Biodivers Conserv 25(1):151–167
Vogt K, Zimmermann F, Kölliker M, Breitenmoser U (2014) Scent marking behaviour and social dynamics in a wild population of Eurasian lynx (Lynx lynx). Behav Process 106:98–106
Watson JEM et al (2018) The exceptional value of intact forest ecosystems. Nat Ecol Evol 2:599–610
Wood CM, Peery MZ (2022) What does “occupancy” mean in passive acoustic surveys? Ibis 164:1295–1300
Wood CM, Popescu VD, Klinck H, Keane JJ, Gutiérrez RJ, Sawyer SC, Peery MZ (2019) Detecting small changes in populations at landscape scales: a bioacoustic site-occupancy framework. Ecol Indic 98:492–507
Wood CM, Klinck H, Gustafson M, Keane JJ, Sawyer SC, Gutiérrez RJ, Peery MZ (2021a) Using the ecological significance of animal vocalizations to improve inference in acoustic monitoring programs. Biol Conserv 35(1):336–345
Wood CM, Kryshak N, Gustafson M, Hofstadter DF, Hobart BK, Whitmore SA, Dotters BP, Roberts KN, Keane JJ, Sawyer SC, Gutiérrez RJ, Peery MZ (2021b) Density dependence influences competition and hybridization at an invasion front. Divers Distrib 27(5):901–912
Yule DL (2000) Comparison of horizontal acoustic and Purse-Seine estimates of Salmonid densities and sizes in eleven Wyoming waters. N Am J Fish Manag 20:759–775
Acknowledgements
The ARUs (Audiomoth) were provided by the University of Turku and GPS tags by Mont Avic Natural Park (Champdepraz, Aosta Valley, Italy). We are also grateful to Jacopo G. Cecere (Italian Institute for Environmental Protection and Research—ISPRA) for technical and practical advice on GPS tags deployment throughout the field season. Thanks to the ELY-keskus Varsinais-Suomi for the licence of deploying loggers on the pygmy owls. We thank Ida Penttinen for great help in visual inspection of the spectrograms.
Funding
Open Access funding provided by University of Turku (UTU) including Turku University Central Hospital. JH was supported by the Operational Programme Research, Development and Education, the Call International Mobility of Researchers—MSCA– IF CZ.02.2.69/0.0/0.0/19_074/0016231, and by the Charles University Research Centre program No. 204069.
Author information
Authors and Affiliations
Contributions
DB, JB and TL contributed to the study conception and design. DB conducted field data collection. Bioacoustic analysis were performed by TR, MG and DB. Habitat analyses were performed by DB, JH, JB and TL. The first draft of the manuscript was written by DB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baroni, D., Hanzelka, J., Raimondi, T. et al. Passive acoustic survey reveals the abundance of a low-density predator and its dependency on mature forests. Landsc Ecol 38, 1939–1954 (2023). https://doi.org/10.1007/s10980-023-01667-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-023-01667-1