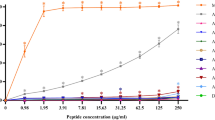
Abstract
The designer proline-rich antimicrobial peptide A3-APO and its Chex1-Arg20 single chain in vivo metabolite were studied for their ability to induce bacterial resistance upon repeated incubation of Escherichia coli and Klebsiella pneumoniae strains in sublethal concentrations. While no resistant E. coli phenotype emerged to either peptides, after 10 passages the K. pneumoniae strain became resistant to the monomer but not the dimer. The major microbiological difference between A3-APO and Chex1-Arg20 is the improved membrane-disintegrating ability of the dimeric prodrug. Thus, in agreement with earlier studies, the induced resistance likely resides in some membrane component rather than the intracellular target protein DnaK. In support, no genetic alteration in the DnaK multihelical lid region could be observed in any of the sensitive or resistant bacterial strains.






Similar content being viewed by others
Abbreviations
- AMP:
-
Antimicrobial peptide
- ATP:
-
Adenosine triphosphate
- cfu:
-
Colony forming units
- MIC:
-
Minimal inhibitory concentration
- PCR:
-
Polymerase chain reaction
References
Amsterdam D (1996) Susceptibility testing of antimicrobials in liquid media. In: Lorian V (ed) Antibiotics in laboratory medicine. Lippincott Williams and Wilkins, Philadelphia, pp 52–111
Bonnet M, Rafi MM, Chikindas ML, Montville TJ (2006) Bioenergetic mechanism for nisin resistance, induced by the acid tolerance response of Listeria monocytogenes. Appl Environ Microbiol 72:2556–2563
Bower MA, Cudic M, Campbell W, Wade JD, Otvos L Jr (2004) Walking the fine line between intracellular and membrane activities of antibacterial peptides. Lett Pept Sci 10:463–473
Bush K, Macielag M, Weidner-Wells M (2004) Taking inventory: antibacterial agents currently at or beyond Phase I. Curr Opin Microbiol 7:466–476
Cassone M, Vogiatzi P, LaMontagna R et al (2008) Scope and limitations of the designer proline-rich antibacterial peptide dimer, A3-APO, alone or in synergy with conventional antibiotics. Peptides 29:1878–1886
Cudic M, Condie BA, Weiner DJ et al (2002) Development of novel antibacterial peptides that kill resistant isolates. Peptides 23:2071–2083
Cudic M, Lockatell CV, Johnson DE, Otvos L Jr (2003) In vitro and in vivo activity of a designed antibacterial peptide analog against uropathogens. Peptides 24:807–820
Ge Y, MacDonald DL, Holroyd KJ, Thornsberry C, Wexler H, Zasloff M (1999) In vitro properties of pexiganan, an analog of magainin. Antimicrob Agents Chemother 43:782–788
Gennaro R, Zanetti M, Benincasa M, Podda E, Minai M (2002) Pro-rich antimicrobial peptides from animals: structure, biological functions and mechanism of action. Curr Pharm Des 8:763–778
Gunn JS, Miller SI (1996) PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol 178:6857–6864
Gutsmann T, Hagge SO, David A et al (2005) Lipid-mediated resistance of gram-negative bacteria against various pore-forming antimicrobial peptides. J Endotoxin Res 11:167–173
Jang WS, Kim CH et al (2003) Biological activities of synthetic analogs of halocidin, an antimicrobial peptide from the tunicate Halocynthia aurantium. Antimicrob Agents Chemother 47:2481–2486
Kindrachuk J, Paur N, Reiman C, Scruten E, Napper S (2007) The PhoQ-activating potential of antimicrobial peptides contributes to antimicrobial efficacy and is predictive of the induction of bacterial resistance. Antimicrob Agents Chemother 51:4374–4381
Kragol G, Lovas S, Varadi G, Condie BA, Hoffmann R, Otvos L Jr (2001) The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry 40:3016–3026
Kragol G, Hoffmann R, Chattergoon MA et al (2002) Identification of crucial residues for the antibacterial activity of the proline-rich peptide, pyrrhocoricin. Eur J Biochem 269:4226–4237
Lai Y, Villaruz AE, Li M, Cha DJ, Sturdevant DE, Otto M (2007) The human anionic antimicrobial peptide dermicidin induces proteolytic defence mechanisms in staphylococci. Mol Microbiol 63:497–506
Lee JY, Yang ST, Lee SK et al (2008) Salt-resistant homodimeric bactenecin, a cathelicidin-derived antimicrobial peptide. FEBJ J 275:3911–3920
Llobet E, Tomas JM, Benoechea JA (2008) Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology 154:3877–3886
Manson JM, Keis S, Smith JM, Cook GM (2004) Acquired bacitracin resistance in Enterococcus faecalis is mediated by an ABC transporter and a novel regulatory protein, BcrR. Antimicrob Agents Chemother 48:3743–3748
Mathur J, Davis BM, Waldor MK (2007) Antimicrobial peptides activate the Vibrio cholera sigmaE regulon through an OmpU-dependent signaling pathway. Mol Microbiol 63:848–858
Mattiuzzo M, Bandiera A, Gennaro R et al (2007) Role of the Escherichia coli SbmA in the antimicrobial activity of proline-rich peptides. Mol Microbiol 66:151–163
McPhee JB, Bains M, Winsor G et al (2006) Contribution of the PhoP-PhoQ and PmrA-PmrB two-component regulatory systems to Mg2+-induced gene regulation in Pseudomonas aeruginosa. J Bacteriol 188:3995–4006
Noto PB, Abbadessa G, Cassone M et al (2008) Alternative stabilities of a proline-rich antibacterial peptide in vitro and in vivo. Protein Sci 17:1249–1255
O’Leary PD, Hughes RA (2003) Design of potent peptide mimetics of brain-derived neurotrophic factor. J Biol Chem 278:25738–25744
Otvos L Jr, Insug O, Rogers ME et al (2000) Interaction between heat shock proteins and antimicrobial peptides. Biochemistry 39:14150–14159
Otvos L Jr, Snyder C, Condie BA, Bulet P, Wade JD (2005) Chimeric antimicrobial peptides exhibit multiple modes of action. Int J Pept Res Ther 11:29–42
Otvos L Jr, de Olivier Inacio V, Wade JD, Cudic P (2006) Prior antibacterial peptide-mediated inhibition of protein folding in bacteria mutes resistance enzymes. Antimicrob Agents Chemother 50:3146–3149
Pini A, Falciani C, Bracci L (2008) Branched peptides as therapeutics. Curr Protein Pept Sci 9:468–477
Tzeng YL, Ambrose KD, Zughaier S et al (2005) Cationic antimicrobial peptide resistance in Neisseria meningitidis. J Bacteriol 187:5387–5396
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cassone, M., Frith, N., Vogiatzi, P. et al. Induced Resistance to the Designer Proline-rich Antimicrobial Peptide A3-APO does not Involve Changes in the Intracellular Target DnaK. Int J Pept Res Ther 15, 121–128 (2009). https://doi.org/10.1007/s10989-009-9176-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-009-9176-1