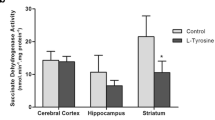
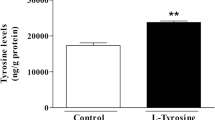
Abstract
Tyrosine levels are abnormally elevated in tissues and physiological fluids of patients with inborn errors of tyrosine catabolism, especially in tyrosinemia type II, which is caused by deficiency of tyrosine aminotransferase and provokes eyes, skin, and central nervous system disturbances. Considering that the mechanisms of brain damage in these disorders are poorly known, in this study, we investigated the in vivo and in vitro effects of tyrosine on some parameters of energy metabolism in cerebral cortex of 14-day-old Wistar rats. We observed that 2 mM tyrosine inhibited in vitro the pyruvate kinase (PK) activity and that this inhibition was prevented by 1 mM reduced glutathione with 30, 60, and 90 min of preincubation. Moreover, administration of tyrosine methyl ester (TME) (0.5 mg/g of body weight) decreased the activity of PK and this reduction was prevented by pre-treatment with creatine (Cr). On the other hand, tyrosine did not alter adenylate kinase (AK) activity in vitro, but administration of TME enhanced AK activity not prevented by Cr pre-treatment. Finally, TME administration decreased the activity of CK from cytosolic and mitochondrial fractions and this diminution was prevented by Cr pre-treatment. The results suggest that tyrosine alters essential sulfhydryl groups necessary for CK and PK functions, possibly through oxidative stress. In case this also occurs in the patients, it is possible that energy metabolism alterations may contribute, along with other mechanisms, to the neurological dysfunction of hypertyrosinemias.







Similar content being viewed by others
References
Natt E, Westphal EM, Toth-Fejel SE, Magenis RE, Buist NR, Rettenmeier R, Scherer G (1987) Inherited and de novo deletion of the tyrosine aminotransferase gene locus at 16q22.1–q22.3 in a patient with tyrosinemia type II. Hum Genet 77:352–358
Scott CR (2006) The genetic tyrosinemias. Am J Med Genet Part C 142C:121–126
Buist NR, Kennaway NG, Fellman JH (1995) Tyrosinaemia type II. In: Bickel H, Wachtel V (eds) Inherited diseases of aminoacid metabolism, 1st edn. Georg Thieme Verlag, Stuttgart, pp 203–235
Rice DN, Houston IB, Lyon IC, Macarthur BA, Mullins PR, Veale AM, Guthrie R (1989) Transient neonatal tyrosinaemia. Inherit Metab Dis 12:13–22
Shasi VK, Pratap RMP, Rao SL (1997) Inhibition of tyrosine aminotransferase by beta-N-oxalyl-l-alpha, beta-diaminopropionic acid, the Lathyrus sativus neurotoxin. J Neurochem 68:2477–2484
Sener RN (2005) Tyrosinemia-computed tomography, magnetic resonance imaging, diffusion magnetic resonance imaging, and proton spectroscopy findings in the brain. J Comput Assist Tomogr 29:323–325
Sgaravatti AM, Vargas BA, Zandoná BR, Deckmann KB, Rockenbach FJ, Moraes TB, Monserrat JM, Sgarbi MB, Pederzolli CD, Wyse ATS, Wannmacher CMD, Wajner M, Dutra-Filho CS (2008) Tyrosine promotes oxidative stress in cerebral cortex of young rats. Int J Dev Neurosci 26:551–559
Sgaravatti AM, Magnusson AS, De Oliveira AS, Rosa AP, Mescka CP, Zanin FR, Pederzolli CD, Wyse AT, Wannmacher CM, Wajner M, Dutra-Filho CS (2009) Tyrosine administration decreases glutathione and stimulates lipid and protein oxidation in rat cerebral cortex. Metab Brain Dis 24:415–425
De Andrade RB, Gemelli T, Rojas DB, Funchal C, Dutra-Filho CS, Wannmacher CMD (2011) Tyrosine inhibits creatine kinase activity in cerebral cortex of young rats. Metab Brain Dis 26:221–227
Willemoes M, Kilstrup M (2005) Nucleoside triphosphate synthesis catalysed by adenylate kinase is ADP dependent. Arch Biochem Biophys 444:195–199
Dzeja PP, Terzic A (2003) Phosphotransfer networks and cellular energetics. J Exp Biol 206:2039–2047
Winterbourn CC, Metodiewa CD (1999) Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med 27:322–328
Jones DP (2006) Redefining oxidative stress. Antioxid Redox Signal 8:1865–1879
Holtzman D, Togliatti A, Khait I, Jensen F (1998) Creatine increases survival and suppresses seizures in the hypoxic immature rat. Pediatr Res 44:410–414
Dechent P, Pouwels PJ, Wilken B, Hanefeld F, Frahm J (1999) Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. Am J Physiol 277(3 Pt 2):R698–R704
Lin Y, Zhang YP, Xiao ZW, Li H, Shen ZW, Chen XK, Huang K, Wu RH (2006) Quantification of brain creatine concentration using PRESS sequence and LCModel: comparison with HPLC method. Conf Proc IEEE Eng Med Biol Soc 1:1928–1931
Feksa LR, Cornelio AR, Vargas CR, Wyse ATS, Dutra-Filho CS, Wajner M, Wannmacher CMD (2003) Alanine prevents the inhibition of pyruvate kinase activity caused by tryptophan in cerebral cortex of rats. Metab Brain Dis 18:129–137
Feksa LR, Cornelio AR, Dutra-Filho CS, Wyse ATS, Wajner M, Wannmacher CMD (2004) Inhibition of pyruvate kinase activity by cystine in brain cortex of rats. Brain Res 1012:93–100
Morre MC, Hefti F, Wurtman RJ (1980) Regional tyrosine levels in rat brain after tyrosine administration. J Neural Transm 49:45–50
Bongiovanni R, Yamamoto BK, Simpson C, Jaskiw GE (2003) Pharmacokinetics of systemically administered tyrosine: a comparison of serum, brain tissue and microdialysate levels in the rat. J Neurochem 87:310–317
Mitchell GA, Grompe M, Lambert M, Tanguay RM (2001) Hypertyrosinemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 1st edn. McGraw-Hill, New York, pp 1977–1982
Leong SF, Lai JCK, Lim L, Clark JB (1981) Energy-metabolising enzymes in brain regions of adult and aging rats. J Neurochem 37:1548–1556
Dzeja PP, Vitkevicius KT, Redfield MM, Burnettm JC, Terzic A (1999) Adenylate kinase-catalyzed phosphotransfer in the myocardium: increased contribution in heart failure. Circ Res 84:1137–1143
Hughes BP (1962) A method for estimation of serum creatine kinase and its use in comparing creatine kinase. Clin Chim Acta 7:597–603
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Dzeja PP, Redfield MM, Burnett JC, Terzic A (2000) Failing energetics in failing hearts. Curr Cardiol Rep 2:212–217
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281:21–40
Saks VA, Khuchua ZA, Vasilyeva EV, Belikova O, Kuznetsov AV (1994) Metabolic compartmentation and substrate channelling in muscle cells. Role of coupled creatine kinases in in vivo regulation of cellular respiration-a synthesis. Mol Cell Biochem 134:155–192
Dzeja PP, Terzic A (1998) Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. Faseb J 12:523–529
Dzeja P, Terzic A (2009) Adenylate kinase and AMP signaling networks: metabolic monitoring, signal communication and body energy sensing. Int J Mol Sci 10:1729–1772
Wolosker H, Panizzutti R, Englender S (1996) Inhibition of creatine kinase by S-nitrosoglutathione. FEBES Lett 392:274–276
Valentini G, Chiarelli LR, Fortin R, Speranza ML, Galizzi A, Mattevi A (2000) The allosteric regulation of pyruvate kinase. J Biol Chem 275:18145–18152
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Azzi A, Gysin R, Kempná P, Munteanu A, Negis Y, Villacorta L, Visarius T, Zingg JM (2004) Vitamin E mediates cell signaling and regulation of gene expression. Ann NY Acad Sci 1031:86–95
Persky AM, Brazeau GA (2001) Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev 53:161–176
Wyss M, Schulze A (2002) Health implications of creatine: can oral creatine supplementation protect against neurological and atherosclerotic disease? Neuroscience 112:243–260
Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A (2001) Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci USA 98:7623–7628
Dzeja PP, Bortolon R, Perez-Terzic C, Holmuhamedov EL, Terzic A (2002) Energetic communication between mitochondria and nucleus directed by catalyzed phosphotransfer. Proc Natl Acad Sci USA 99:10156–10161
Neumann D, Schlattner U, Wallimann T (2003) A molecular approach to the concerted action of kinases involved in energy homoeostasis. Biochem Soc Trans 31:169–174
O’Gorman E, Beutner G, Dolder M, Koretsky AP, Brdiczka D, Wallimann T (1997) The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett 414:253–257
Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T (2006) Cardiac system bioenergetics: metabolic basis of the Frank–Starling law. J Physiol 571(Pt 2):253–273
Dzeja PP, Terzic A (2005) Mitochondrial-nucleus energetic communication: role of phosphotransfer networks in processing cellular information. In: Gibson G, Dienel G (eds) Handbook of neurochemistry and molecular neurobiology, neural energy utilization. Kluwer, New York
Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR (2008) Functions and effects of creatine in the central nervous system. Brain Res Bull 76:329–343
Klein AM, Ferrante RJ (2007) The neuroprotective role of creatine. Subcell Biochem 46:205–243
Koufen P, Stark G (2000) Free radical induced inactivation of creatine kinase: sites of interaction, protection, and recovery. Biochim Biophys Acta 1501:44–50
Magni DV, Oliveira MS, Furian AF, Fiorenza NG, Fighera MR, Ferreira J, Mello CF, Royes LF (2007) Creatine decreases convulsions and neurochemical alterations induced by glutaric acid in rats. Brain Res 1185:336–345
Pucar D, Dzeja PP, Bast P, Gumina RJ, Drahl C, Lim L, Juranic N, Macura S, Terzic A (2004) Mapping hypoxia-induced bioenergetic rearrangements and metabolic signaling by 18O-assisted 31P NMR and 1H NMR spectroscopy. Mol Cell Biochem 256–257:281–289
Prass K, Royl G, Lindauer U, Freyer D, Megow D, Dirnagl U, Stockler-Ipsiroglu G, Wallimann T, Priller J (2007) Improved reperfusion and neuroprotection by creatine in a mouse model of stroke. J Cereb Blood Flow Metab 27:452–459
Rae C, Digney AL, McEwan SR, Bates TC (2003) Oral creatine monohydrate supplementation improves brain performance: a double-blind, placebo-controlled, cross-over trial. Proc Biol Sci 270:2147–2150
Acknowledgments
This study was supported in part by grants from Conselho Nacional de Desenvolvimento Científico e Tecnoloógico (CNPq-Brazil), Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS, RS-Brazil), and Programa de Núcleos de Excelência-Financiadora de Estudos e Projetos (PRONEX II, FINEP-CNPq-Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Andrade, R.B., Gemelli, T., Rojas, D.B. et al. Tyrosine impairs enzymes of energy metabolism in cerebral cortex of rats. Mol Cell Biochem 364, 253–261 (2012). https://doi.org/10.1007/s11010-012-1225-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1225-y